This wiki has undergone a migration to Confluence found Here
Difference between revisions of "Product List"
Jump to navigation
Jump to search
| Line 59: | Line 59: | ||
=====Documents===== | =====Documents===== | ||
*[[Product_CDA | Clinical Document Architecture (CDA)]] | *[[Product_CDA | Clinical Document Architecture (CDA)]] | ||
| − | **CDA R2 [[Product_CDA_R2_IG |Implementation Guides]] | + | **CDA R2 [[Product_CDA_R2_IG_CommonClinDocs |Implementation Guides for Common Clinical Documents]] such as H&P, Progress Note, etc. |
| − | ** | + | **CDA R2 [[Product_CDA_R2_IG |Implementation Guides]] for specialized or unstructured clinical documents |
| − | ** | + | **[[Product_CCD | Continuity of Care Document (CCD)]] |
| + | **[[Product_Claims_Attachments | Claims Attachments]] | ||
=====Services===== | =====Services===== | ||
Revision as of 13:46, 13 October 2010
Contents
- 1 Product List
- 2 Governance and Maintenance
- 3 Suggested Additions
Product List
Product Briefs - origins
- see Product Brief Template
- Microsoft Excel Version (2009-12-17) of Product List
- Product List FAQ
Summary Stats (2010-06-22): Products - 96 (95 having material on wiki)
Steward-reviewed (reviewed by steward Work Group) - 84 (88% complete)
Marketing-reviewed - 52
- List of product category pages
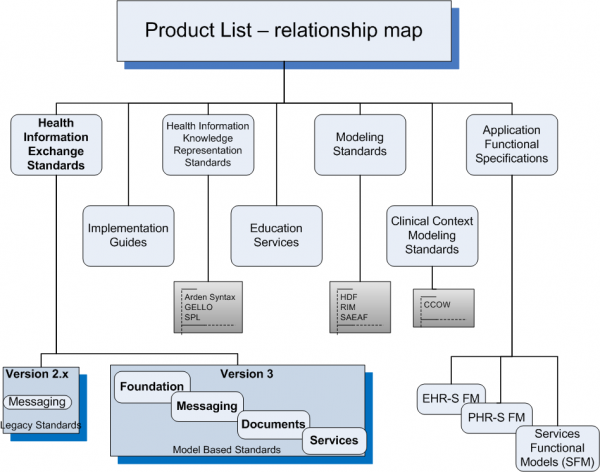
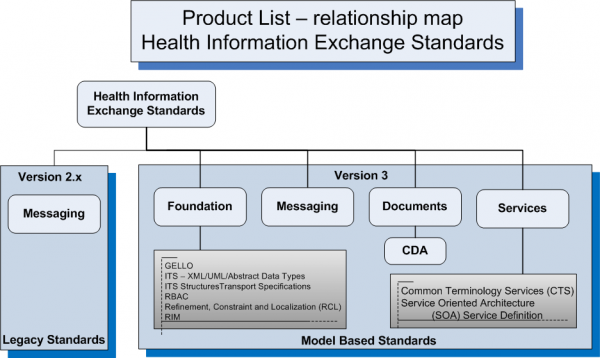
Health Information Exchange Standards
Version 2
Version 3
Foundation
- ANSI/HL7 V3 RIM, R1-2003; HL7 Version 3 Standard: Reference Information Model (RIM), Release 1 - see Modeling Standards
- Vocabulary Specifications- see Modeling Standards
- ANSI/HL7 V3 DT, R1-2004; HL7 Version 3 Standard: Data Types - Abstract Specification, Release 1
- ANSI/HL7 V3 RCL: R1-2003, R2-2007; HL7 Version 3 Standard: Refinement, Constraint, and Localization to version 3 Messages
- XML Implementation Technology Specification (ITS) - Data Types
- ANSI/HL7 V3 XMLITSDT, R1-2004; HL7 Version 3 Standard: XML Implementation Technology Specification - Data Types
- HL7 V3 ITS, R2; XML Implementation Technology Specification R2 Guide, Release 1, Informative 2008May
- HL7 V3 XMLITS_DT1.1, R1; XML ITS Data Types R1.1, Informative 2010Jan
- HL7 V3 ISO DT, R1; R2-2009 ISO Datatypes 2009Sept
- ANSI/HL7 V3 UMLITSDT, R1-2004; HL7 Version 3 Standard: UML Implementation Technology Specification - Data Types - defunct with ISO Datatypes; Work Group considering withdrawal.
- ANSI/HL7 V3 XMLITSDT, R1-2004; HL7 Version 3 Standard: XML Implementation Technology Specification - Data Types
- XML ITS Structures
- ANSI/HL7 V3 XMLITSSTR, R1-2005: HL7 Version 3 Standard: XML Implementable Technology Specification for V3 Structures; XML Structure R1 - 9/26/2005
- HL7 V3 XMLITSSTR1.1, R1; XML Structure R1.1, 2009Sept
- HL7 V3 ITS, R2; XML Structure R2 - 2010May.
- HL7 V3 ITS RIMSR, R1; RIM Serializations R1 - 2010May
- ANSI/HL7 V3 CTS, V1-2005, ISO/HL7 27951:2009(E); HL7 Version 3 Standard: Common Terminology Services, Release 1 -see Services
- ANSI/HL7 V3 GELLO, R2-2010; HL7 Version 3 Standard: GELLO: A Common Expression Language, Release 2- see Knowledge Representation Standards
- Transport Specifications
- ANSI/HL7 V3 TRMLLP, R2-2006; HL7 Version 3 Standard: Transport Specification - MLLP, Release 2
- ANSI/HL7 V3 TR ebXML, R1-2008; HL7 Version 3 Standard: Transport Specification - ebXML, Release 1
- Transport Specification: ISO 9660 Removable Media Release 1
- ANSI/HL7 V3 RBAC, R1-2008; HL7 Version 3 Standard: Role-based Access Control Healthcare Permission Catalog, Release 1
- HL7 V3 MODELS, R1 (3rd Normative Ballot in May 2010) - Core Principles and Properties of V3 Models
- HL7 V3 MIF, R1 (I1 May 2010) - Model Interchange Format (MIF)
Messaging
Documents
- Clinical Document Architecture (CDA)
- CDA R2 Implementation Guides for Common Clinical Documents such as H&P, Progress Note, etc.
- CDA R2 Implementation Guides for specialized or unstructured clinical documents
- Continuity of Care Document (CCD)
- Claims Attachments
Services
- Common Terminology Services (CTS)
- HL7 Version 3 Standard: Privacy, Access and Security Services (PASS)
- HL7 Version 3 Methodology: Service Oriented Architecture; Service Definition, Release 1
Health Information Knowledge Representation Standards
Arden Syntax, SPL, GELLO
Modeling Standards
- Healthcare Development Framework (HDF)
- Reference Information Model (RIM)
- Vocabulary Specifications
- SAIF - the Services-Aware Interoperability Framework, formerly known as SAEAF - Services-Aware Enterprise Architecture Specification
Application Functional Specifications
System Functional Models and Functional Profiles, Service Functional Models
- Electronic Health Record Functional Model (EHR FM)
- HL7 Ambulatory Oncology Functional Profile, in development
- HL7 EHR Behavioral Health Functional Profile
- HL7 EHR Child Health Functional Profile
- HL7 EHR Clinical Research Functional Profile
- HL7 EHR System Long Term Care Functional Profile
- HL7 Records Management and Evidentiary Support Functional Profile
- HL7 Vital Reporting Functional Profile, in development
- DSTU: HL7 EHR Lifecycle FM (EHR/LM), Release 1; ends September 2010
- DSTU: Personal Health Record System Functional Model (PHR-S FM), Release 1; ends December 2010
- (Entity) Identification Service Functional Model (EIS SFM) aka Identity Cross-Reference Service Functional Model (IXS)
- Retrieve Locate Update Service Functional Model (RLUS SFM)
- DSTU: Decision Support Service Functional Model (DSS SFM)
- Healthcare, Community Services and Provider Directory Service Functional Model (SPDIR SFM)
- DSTU: HL7 Version 3 Standard: Regulated Studies; Clinical Research Filtered Query (CRFQ) Service Functional Model (SFM), Release 1; ends May 2010
Clinical Context Management Standards
Implementation Guides
- Implementation/Conformance Work Group Wiki page on Implementation Guides
Education Services
Governance and Maintenance
Ongoing and annual review, maintenance, and endorsement of Product Definitions
- New product request - - TSC must be aware of and recognize on a PSS coming for approval, and add to inventory
- Updates to products: review annually prior to September plenary, check for ANSI 5-year anniversary, etc.
- Evaluate potential for expired products or standards: with DSTU expiration, withdrawal of ANS standard, replacement?
Suggested Additions
- GOM
- HDF
- PLPCD
- MoUs, Charter Agreements, and Liasons -- While these are not “traditional” products – they do cost involve giving away access to HL7 IP and/or meetings and/or brand, and so deliver value and have a cost
- Podcasts (audio and/or Video) from WGMs — these could be delivered as a member benefit which would be hugely valuable to many current and potential members (helping to reduce very expensive member churn). Some extracts or complete items could be delivered free as a marketing exercise
- If we're going to conflate classes and ennumerated instances in the diagram, we should list all of the serivces that have currently been approved, i.e. EIS, RLUS, CRFQ, and (I believe) PASS.
- NCI notes we see the partnership with HL7 going forward as one of HL7 taking the lead on development and maintenance of the core SAEAF content as manifest in the SAEAF Book, a resource for all organizations interested in adopting SAEAF. In turn, the NCI CBIIT plans to develop a set of tools for managing SAEAF-derived semantic content (we lump this content under the rubrique "model/artifact management") which we would be willing to share with the larger community.
- As well as selling membership and WGM attendance, HL7 currently runs a franchising operation. HL7 leases Affiliate rights to the National Organisations in return for a cut of their membership revenue. This should be captured, documented, and managed as a Product. We should be looking at how we maintain this product specification, and how we sell it, how we price it, and how we ensure that it is delivered in a cost effective and high value way. Maybe we will move to a different business model for global presence — but for now there are franchises and we need to include that in our documentation and planning processes