Product BRIDG
Product Brief - Biomedical Research Integrated Domain Group (BRIDG)
Contents
- 1 Product Brief - Biomedical Research Integrated Domain Group (BRIDG)
- 1.1 Product Name
- 1.1.1 Topics
- 1.1.2 Standard Category
- 1.1.3 Integration Paradigm
- 1.1.4 Type
- 1.1.5 Releases
- 1.1.6 Summary
- 1.1.7 Description
- 1.1.8 Business Case (Intended Use, Customers)
- 1.1.9 Benefits
- 1.1.10 Implementations/ Case Studies (Actual Users)
- 1.1.11 Resources
- 1.1.12 Relationship to/ Dependencies on, other standards
- 1.1.13 Links to current projects in development
- 1.1 Product Name
back to Main_Page
back to Product_List
Product Name
Biomedical Research Integrated Domain Group (BRIDG)
Domain Analysis Model (DAM)
Topics
Standard Category
- Health Information Knowledge Representation
Integration Paradigm
Type
Informative, balloting 2010May
Releases
HL7 BRIDG DAM, R1 - Informative ballot in 2010 May
Summary
The Biomedical Research Integrated Domain Group (BRIDG) grew from a concept that began in 2003. The concept was to develop a Domain Analysis Model (DAM) that documented the shared semantics of the protocol-driven-research domain. The project was initiated in March 2004.
The Biomedical Research Integrated Domain Group (BRIDG) Model is a collaborative effort engaging stakeholders from the Clinical Data Interchange Standards Consortium (CDISC), the HL7 Regulated Clinical Research Information Management Work Group (RCRIM), the National Cancer Institute (NCI) and its Cancer Biomedical Informatics Grid (caBIG®), and the US Food and Drug Administration (FDA). The BRIDG model is an instance of a Domain Analysis Model (DAM). The goal of the BRIDG Model is to produce a shared view of the dynamic and static semantics for the domain of protocol-driven research and its associated regulatory artifacts. This domain of interest is further defined as:
Protocol-driven research and its associated regulatory artifacts,
i.e. the data, organization, resources, rules, and processes involved in the formal assessment of the utility, impact, or other pharmacological, physiological, or psychological effects of a drug, procedure, process, subject characteristic, or device on a human, animal, or other subject or substance plus all associated regulatory artifacts required for or derived from this effort, including data specifically associated with post-marketing adverse event reporting.
Description
The goal of the BRIDG Project is to develop a shared view of the data, relationships, and processes which collectively define the domain of “protocol-driven research and its associated regulatory artifacts.” Specifically, the formal definition of the domain-of-interest for the BRIDG Project stakeholders is:
Protocol-driven research and its associated regulatory artifacts,
i.e. the data, organization, resources, rules, and processes involved in the formal assessment of the utility, impact, or other pharmacological, physiological, or psychological effects of a drug, procedure, process, subject characteristic, or device on a human, animal, or other subject or substance plus all associated regulatory artifacts required for or derived from this effort, including data specifically associated with post-marketing adverse event reporting. A shared view of the various data structures and processes that define the BRIDG Model’s domain-of-interest is essential in achieving the larger goal of semantic interoperability (SI) both among people (human semantic interoperability (HSI)) and systems (computable semantic interoperability (CSI)). Through the explicit definitions of shared semantics, either HSI or CSI (depending on the desired goal in a particular context) is possible both within the BRIDG domain-of-interest and between the BRIDG domain and other ‘intersecting’ domains (e.g. Public Health, healthcare, etc.).
With the development of BRIDG release 3.0 the model was sectioned into three layers:
- A sub-domain specific layer, allowing domain experts to see their semantics in the 5 user-friendly sub-domain models: Protocol Representation, Study Conduct, Adverse Event, Regulatory, Common;
- A comprehensive layer that combines all the sub-domains in a single view of the harmonized and shared semantics;
- A RIM-based layer that represents the BRIDG concepts but uses the RIM classes and HL7 diagrammatic representation for the HL7-savvy technical personnel.
BRIDG 3.0 also includes tags on model elements identifying the source model element(s) from which they were derived, a revision to the Study Design portion of the model to make it more flexible, the adoption of a UML style guide, and an updated User's Guide.
The BRIDG static content, by release, is outlined in Figure 2 below.
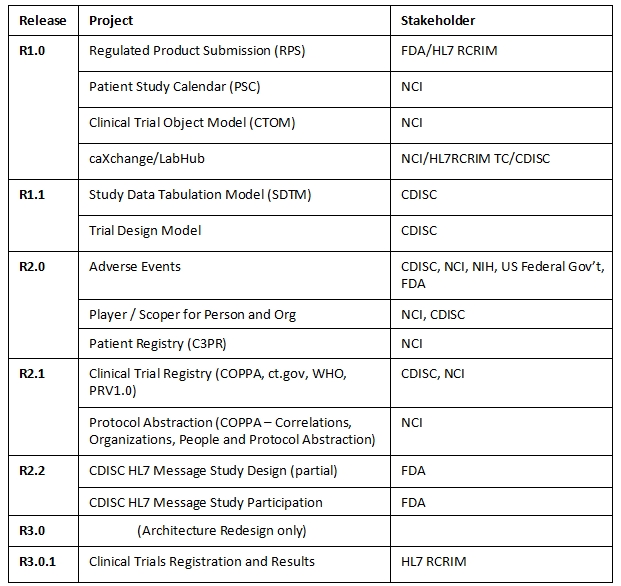
THE PURPOSE OF THE BRIDG The goal of the BRIDG Project is to produce a shared view of the dynamic and static semantics of a common domain-of-interest, specifically the domain of protocol-driven research and its associated regulatory artifacts.
Business Case (Intended Use, Customers)
A shared semantic view is essential if the clinical research community, both for itself and also as part of the larger Healthcare and life sciences communities, is to achieve computable semantic interoperability (CSI), i.e. the ability for information systems to exchange at a machine-to-machine level the meaning (rather than simply the structure) of data and/or to effectively combine functionality across machine/system boundaries. Stated another way, in order to realize the various data interchange and application interactions that are known by members of the BRIDG stakeholder organizations to be requirements for CSI, a shared view of dynamic and static semantics must be established.
Benefits
Implementations/ Case Studies (Actual Users)
The BRIDG Project is a collaborative effort engaging stakeholders from four organizations:
- Clinical Data Interchange Standards Consortium (CDISC)
- HL7 Regulated Clinical Research Information Management Working Group (HL7 RCRIM WG)
- National Cancer Institute (NCI), including the Cancer Biomedical Informatics Grid (caBIG™) project
- Food and Drug Administration (FDA)
Resources
Work Groups
Education
- BRIDG Web Site http://www.bridgmodel.org/
- BRIDG Project Site http://gforge.nci.nih.gov/projects/bridg-model/
- BRIDG Release Site http://gforge.nci.nih.gov/frs/?group_id=342
- BRIDG as a DAM wiki http://wiki.hl7.org/index.php?title=BRIDG_as_DAM
BRIDG User Guide is available as a part of the release packages from the BRIDG release site above
- See more at http://www.hl7.org/implement/training.cfm
Relationship to/ Dependencies on, other standards
Links to current projects in development
- Project Insight ID # 538, BRIDG (Biomedical Research Integrated Domain) Domain Analysis Model (DAM)