201709 Implantables Tracking
Track Name
Point-of-care Medical Device and Implantable Tracking
Submitting WG/Project/Implementer Group
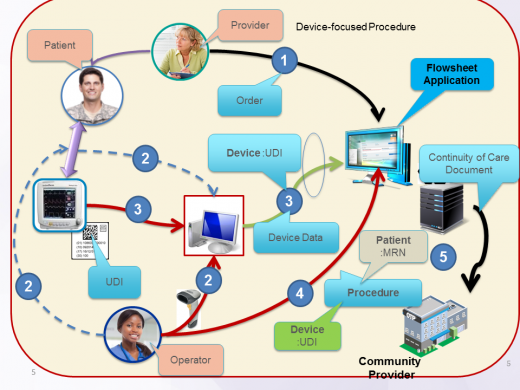
This track provides a set of simple test cases for Tracking Medical Devices (i.e. diagnostic, therapeutic, monitoring) and Implantables (biologics/tissue/cells and non-biologics/life-supporting/life-sustaining) at point of care addresses current problems relate to information accuracy and it provides procedure contextual information. This track specifies test cases related FHIR STU3 Procedure and Device resources used to report information from the point-of-care to enterprise system consistent with the IHE PCC Point-of-care Medical Device Tracking Supplement.
Justification
- Implantable medical devices are costly and concerns about illegitimate (i.e., counterfeit, stolen) products has become a global issue
- By 2021 all medical devices that may be used in a point-of-care procedure will be labeled using FDA UDI that will also relate to software/firmware release
- Post-market surveillance of implantable medical devices can be challenging
- Implantable medical device adverse events and recalls pose a patient safety issue
- Acquiring medical device data used at the point-of-care is difficult to retrieve for reuse at a later time
- A consistent device identification scheme closes the loop on data acquisition at the point-of-care to support reporting of medical device data
- Medical device data used for:
- Continuum of care (e.g., Discharge Summary, Referrals)
- Registries (e.g., Total Joint Registry)
- Payers (e.g., government provided, private insurance)
- Can associate a medical device used for monitoring a disease or symptom of a disease (e.g., vital sign monitors, pulse oximeters, blood glucose monitors) to a patient for querying the device or procedure using the UDI
- Increase patient safety
- Traceability of medical devices (avoid use of counterfeit or illegitimate products)
- Quality issues identified (e.g., recalls, adverse events)
- Increase accurate medical device data capture at the point-of-care
- Eliminates human error from manual medical device data entry
Proposed Track Lead
Ioana Singureanu, VA/BZI, Skype: ioanasingureanu
Expected participants
- Department of Veterans Affairs - "Medical DeviceTracker"
- Magpie360 - "Medical DeviceTracker"
- Eversolve, LLC: "Medical Device Server"
Roles
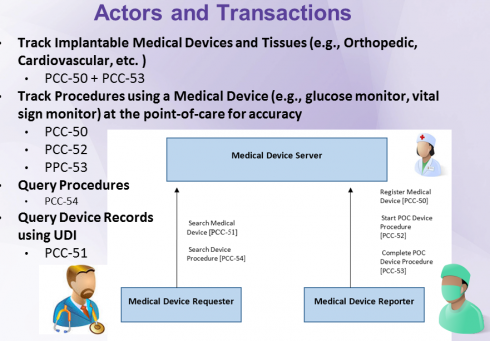
The test roles for this track are based on the IHE PCC PMDT Actors below:
Point-of-Care Medical Device Reporter
- Point-of-care client; it registers medical device, creates/updates point-of-care procedure information, query patient based on identifier scanned at the point-of-care. This role is played by a system used to track information about medical devices used at the point-of-care. The device and procedure information are populated at the point-of-care using scanned (AIDC) UDI and patient identifier to simplify the accurate tracking of this information.
Medical Device Server
- FHIR server, exposes Device, Procedure - references Patient. It is used to persist device and procedure information originating at the point of care. This information is made available to other information systems in the enterprise. This role may be played by a Medical Device Registry or an EHR system.
Point-of-Care Medical Device Requester
- This role is played by any information system that requires to compile an implantable device list for patient, evaluate outcomes based on device type (i.e. DI), respond manufacturers' recalls, and address patient safety events.
Scenarios
The following is a summary test cases proposed for
- Register (create) device record using
- UDI and DI only
- UDI and DI and optional attributes
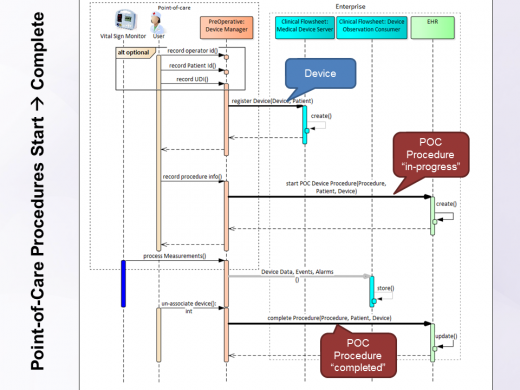
- Start procedure at the point of care. This may an infusion procedure, a diagnostic procedure using a device, a monitoring session, etc. The Procedure resource specifies the status as "in-progress" and the start of the procedure as well as the coded procedure type, patient, practitioner, etc. At the minimum the patient, the device, and the practitioner known at the point-of-care should be specified.
- with externally referenced device
- Complete procedure; it marks its status to "completed" and specifies the time it was completed.
- with externally referenced device
- Complete implantable procedure; this a one-time record that a device, tissue, or tissue products was implated.
- with externally referenced device; it assumes the device was already registered and its UDI and its other identifiers were captures.
- with contained device reference; the device information is included as "contained" resource in the Procedure.
- Search procedures; to compute outcomes, compile implantable device lists for transition-of-care, to respond to recall notifications
- by patient
- by other optional search parameters
- Search devices; to track device utilization, for quality control, to compute outcomes, compile implantable device lists for transition-of-care, to respond to recall notifications
- by udi-carrier
- by patient
- by udi-di
Register Medical Device or Implantable from the point-of-care
- Action: The 'Tracker' creates a
Deviceresource (using PUT/update), setsDevice.idto a 'GUID',Device.udi.carrierAIDCto the base64-encoded, 'scanned UDI', setsDevice.statusto 'active'. TheDevice.patientreferences is set to external reference previously retrievedPatientresource. (see 'Precondition'). - Precondition: The 'Tracker' queried the
Patientresource using thePatient.identifierscanned at the point-of-care. This external references is used inDevice.patient. - Success Criteria: The 'Requester' queries
Deviceby 'udi-carrier' (expression:Device.udi.carrierHRF | Device.udi.carrierAIDC) and retrieves one matching resource containing the correct information. The 'Tracker' can retrieve it by logical id/GUID. - Bonus point: specify optional data elements (S, 0..) of the
Deviceresource.
Complete an Implantable Procedure
- Action: The 'Tracker' creates a
Procedureresource (using PUT/update), sets theProcedure.idto a GUID,Procedure.statusto 'completed',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedDateTimeto the current date/time. The 'Tracker' specifies theProcedure.codecorresponding the implant procedure performed. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Requester' searches the
Procedureresource based onProcedure.code(expression:code).
Start a Point-of-Care Procedure
- Action: The 'Tracker' creates a
Procedureresource (using PUT/update), sets theProcedure.idto GUID,Procedure.statusto 'in-progress',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedPeriod.startto the current date/time. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Requester' searches the
Procedureresource based onProcedure.code(expression:code).
Complete a Point-of-Care Procedure
- Action: The 'Tracker' updates the
Procedureresource (using PUT/update), it sets theProcedure.idto its previously set GUID,Procedure.statusto 'completed',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedPeriod.endto the current date/time. The 'Tracker' specifies theProcedure.codecorresponding the implant procedure performed. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. The 'Tracker' created a first version of theProcedure - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Tracker' submits the same transaction using a "contained"
Deviceresource.
TestScript(s)
- TestScript resources will be created/registered for Jan FHIR Connectathon
Outcomes
- Persisted Device resources and procedures performed at the point-of-care for implants (i.e. Complete POC Procedure) in a new FHIR server.
- We need additional test cases for replacing an implant (i.e. marking the current Device as "inactive") with another implant and documenting the associated new Implant Procedure.
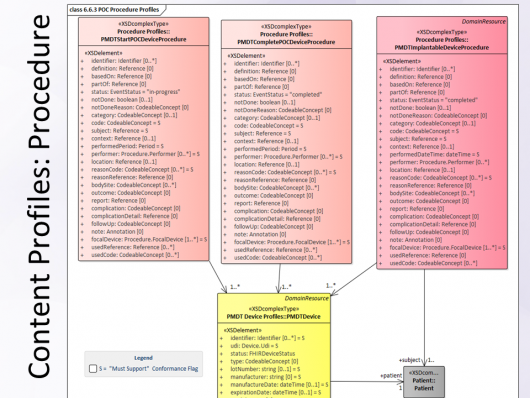
- Updated several "must support" elements and created the PMDT Device resource. In its next iteration this track will use
- ImplementationGuide for the overall PMDT integration profile,
- StuctureDefinition resources for each profiles
- Point-of-Care Device profile,
- Point-of-Care Procedure Start,
- Point-of-Care Procedure Complete,
- Complete Implant Procedure.
- This track will repeat at the Jan WGM
- The test cases will be further refined for both the FHIR and IHE Connectathon
- This track will be repeated in January 2018 during the 201801 Medical Device and Implantables Tracking using UDI.