Difference between revisions of "SubstanceDefinition FHIR Resource Proposal"
Riksmithies (talk | contribs) |
Riksmithies (talk | contribs) |
||
| Line 82: | Line 82: | ||
==Content sources== | ==Content sources== | ||
| − | + | The original basis for the resource is the information in ISO 11238 Substances standard. A large amount of data exists in the FDA GSRS implementation of ISO 11238. | |
| − | + | EMA has well developed requirements in this space, which have influenced the current draft resource. They have a FHIR API ready to be implemented (Q3 2019) that uses this resource, to cover those requirements. | |
==Example Scenarios== | ==Example Scenarios== | ||
Revision as of 23:38, 1 July 2019
Contents
- 1 SubstanceDefinition
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 RIM scope
- 1.7 Resource appropriateness
- 1.8 Expected implementations
- 1.9 Content sources
- 1.10 Example Scenarios
- 1.11 Resource Relationships
- 1.12 Timelines
- 1.13 gForge Users
- 1.14 When Resource Proposal Is Complete
- 1.15 FMG Notes
SubstanceDefinition
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019
Contributing or Reviewing Work Groups
- Pharmacy
- O&O
FHIR Resource Development Project Insight ID
1367, 1338
Scope of coverage
To support the detailed definition of substances, including to molecular level, manufacturing processes and ingredients. To cover the content areas of the ISO 11238 IDMP Substance standard, and other domain areas with similar requirements.
RIM scope
Similar in scope to the substance parts of CPM. Entity: Material (EntityClass="MAT")
Resource appropriateness
There is an upcoming requirement to support the standardised exchange of detailed definitional Substance data (as covered by the ISO 11238 specification).
This resource does not intend to clash with the existing Substance FHIR resource, but complements with an extra level of detail. It is seen as a sibling rather than a parent or a superclass to be profiled.
It is intended to add an extra level of substance detail - beyond what is covered for day to day prescribing by the Substance resource - such as is typically used by regulators. This data is only indirectly used during normal medication related work flows (e.g. for occasional look-ups of unfamiliar substances).
Manufacturers of medicinal products submit this detailed substance data to regulators. When they seek authorization for a new product, they submit details of all the ingredient substances. They re-submit when substance parameters change (ingredient manufacturer change, manufacturing process etc).
This is implemented EU-wide with the current XEVMPD standard, and there is a wish to move to FHIR for the EMA "SPOR" system currently in development. The substance catalogue (pre-registration of substances to be later used as ingredients) is intended to be the first part to go live.
This resource can also be used when downloading substances information from repositories such as the FDA Ginas system (G-SRS). This system acts as a "wikipedia" for substances, with structured and very detailed information (currently using a proprietary JSON format). Regulators also exchange this information between each other, to synchronise substance catalogues.
Expected implementations
EMA - Implementation as part of the EU-wide SPOR system. The substance catalogue is the "S" of SPOR (Substances, Products, Organizations, Referentials).
FDA - Drug Manufacturing Quality information (aka PQ/CMC, Pharmaceutical Quality). Specific plans to use this resource for that project.
FDA - Ginas/G-SRS project (Global Ingredient Archival System). This has already implemented a proprietary message solution, with similar scope to what is planned for FHIR
Content sources
The original basis for the resource is the information in ISO 11238 Substances standard. A large amount of data exists in the FDA GSRS implementation of ISO 11238.
EMA has well developed requirements in this space, which have influenced the current draft resource. They have a FHIR API ready to be implemented (Q3 2019) that uses this resource, to cover those requirements.
Example Scenarios
Substance definitions for the various categories (Chemical, Polymer, Protein etc). Use of "Specified Substance" area of 11238 to add extra information around manufacturing process etc.
Resource Relationships
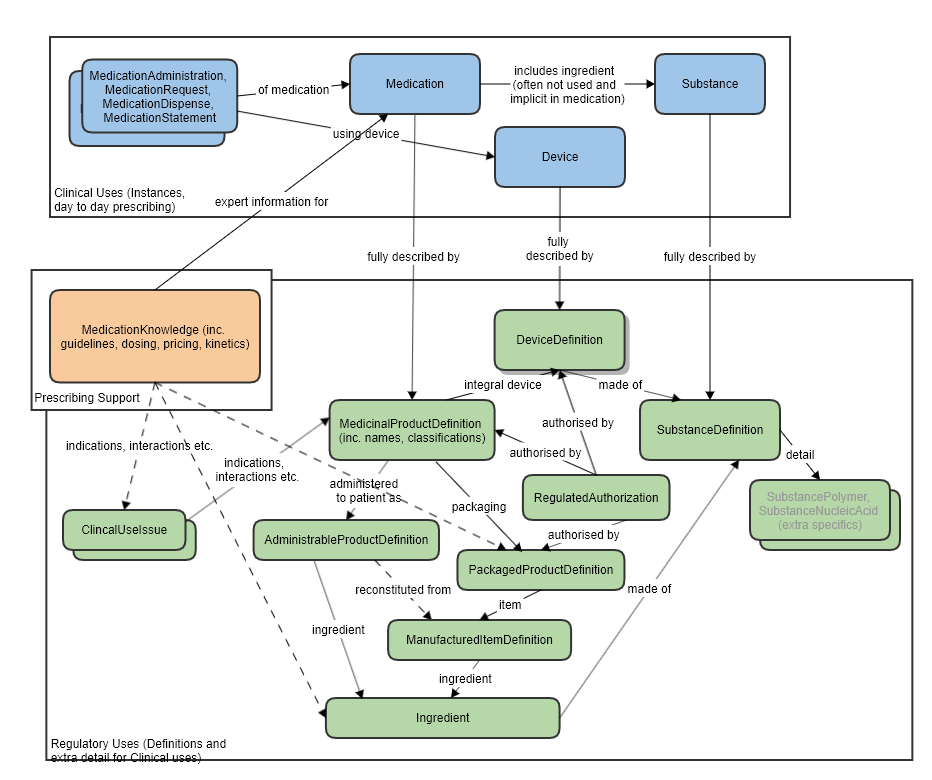
See diagram below and also associated proposal: MedicinalProduct_FHIR_Resource_Proposal
Reference to Organization, for the manufacturer.
SubstanceDefinition and Substance
There is expected to be a reference from Substance to SubstanceDefinition, to be able to point to a more detailed definition. This may be an actual FHIR reference, or, perhaps more likely, it could operate via Substance.Code, which would correspond to SubstanceDefinition.code.
This resource is not expected to replace the existing Substance resource - which is limited in scope to the small number of items needed to support direct clinical/medicinal use. (The Substance resource could be considered to be a "Substance Use".)
SubstanceDefinitionis a collection of definitional information that an instance of a Substance resource (e.g. in a Medication, within a MedicationDispense) can refer to.
It is not expected that a SubstanceDefinitionwould ever directly substitute for the use of Substance.
In theory, all the SubstanceDefinitioninformation could be contained in a hugely expanded Substance resource (and then the existing Substance scope could be profiled back out again). But this would create an unmanageably large Substance resource for the very common and narrow medical/clinical use cases.
Since the key regulatory use of SubstanceDefinitionis almost orthogonal to the clinical use cases, it seems appropriate to have the SubstanceDefinition resource available as a sibling to Substance, not a parent. The SubstanceDefinition resource would also be used on its own, unrelated to any Substance instance, in those regulatory and drug information use cases.
Overlap between SubstanceDefinition and Substance
In practical terms there very little overlap of attributes between SubstanceDefinition and Substance. The Substance resource has only a few fields that are definitional (which are the only ones that would overlap).
Identifier and status are specific to this substance instance.
Category is definitional, and so does overlap, but could also be a local classification.
Code is the link between the two, and description could be considered a very small (possibly local) summary of the whole SubstanceDefinition, and so be a useful overlap.
The Instance component is not definitional so does not clash.
The Ingredient component is primarily for cases when the main Substance is made up on-demand - mixed rather than manufactured - and so would not itself be covered by a global SubstanceDefinition (hence no overlap).
SubstanceDefinition and Medication
The Medication resource has a reference to Substance. That is not expected to change. As described, SubstanceDefinition is additional, definitional information about substances. It is not itself expected to be used as a substance instance - which is what Substance is for.
SubstanceDefinition and Device
There is no direct relationship between SubstanceDefinition and Device anticipated.
In theory, since devices are made of substances, it would be possible, as with any physical object, to use a SubstanceDefinition to describe the details of materials that comprise it. However this is not a currently proposed use case and device material is not supported by the existing Device resource.
A proposed DeviceSpecification resource would likely use SubstanceDefinition to define its materials.
Timelines
Early draft by December 2017 comment-only ballot.
gForge Users
riksmithies (already has commit permission)
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org