Difference between revisions of "CSCR-119 create CMET to cover Rx Device requirements (assignedDevice etc) (Rx harmonization)"
Riksmithies (talk | contribs) |
Riksmithies (talk | contribs) |
||
| Line 24: | Line 24: | ||
[[File:CSDeviceCMET.png]] | [[File:CSDeviceCMET.png]] | ||
| + | |||
| + | Update 8th Feb - note that Pharmacy are discussing device in their models so check outcome before implementing | ||
== Rationale == | == Rationale == | ||
Latest revision as of 18:50, 8 February 2013
Back to Clinical Statement Change Requests page.
| Submitted by: Rik Smithies | Revision date: <<Revision Date>> |
| Submitted date: 09-Jan-2013 | Change request ID: CSCR-119 |
Issue
From Rx alignment sheet (posted to list 9th Jan)
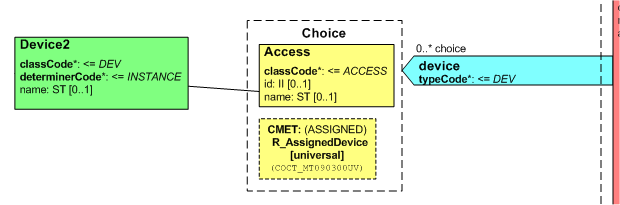
Pharmacy devices are a choice of R_AssignedDevice (same thing on both DMIM and Medication Order) or an ACCESS role with a basic device on it. This is all attached to SBADM. Pictured below.
CS has DEV on a PROC but not on SBADM, and it only allows common product model things. R_AssignedDevice is nothing like CPM, so this cannot cover it even if extended to also be off SBADM.
Recommendation
To cover this in CS need to add what is in Rx, as shown below. Suggest make a dedicated CMET CSRxAdministrationDevice to cover this, with that content, attached to SBADM.
Update 8th Feb - note that Pharmacy are discussing device in their models so check outcome before implementing
Rationale
Discussion
Recommended Action Items
Resolution
Motion to accept: Stephen, Hans Against: 0; Abstain: 1; In Favor: 8 carried