This wiki has undergone a migration to Confluence found Here
CSCR-072 Device Participation
Jump to navigation
Jump to search
Back to Clinical Statement Change Requests page.
| Submitted by: Austin Kreisler | Revision date: <<Revision Date>> |
| Submitted date: 12/17/2007 | Change request ID: CSCR-072 |
Issue
For some laboratory testing we have identified a requirement to communicate what testing kit was used to create a laboratory observation. There can also be test kit specific interpretation ranges associated with laboratory results.
Recommendation
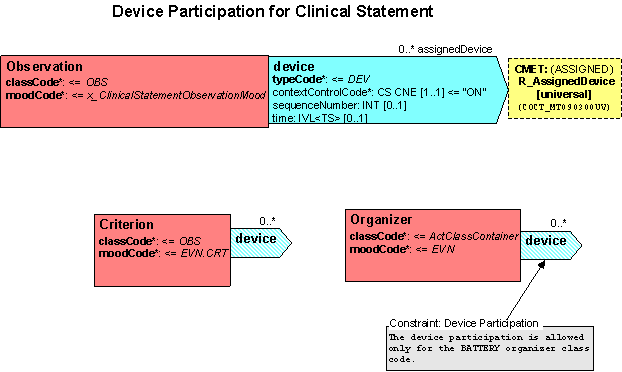
- Add a device (DEV) participation from Observation to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are:
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” sequenceNumber (optional) INT [0..1] time (optional) IVL<TS> [0..1]
- Add a device (DEV) participation from Criterion to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are:
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” sequenceNumber (optional) INT [0..1] time (optional) IVL<TS> [0..1]
Rationale
Discussion
According to the RIM definition for Observation.methodCode, documentation the test kits used should be handled as a device participation on the observation. From the RIM definition of Observation.methodCode: "...The methodCode should not be used to identify the specific device or test-kit material used in the observation. Such information about devices or test-kits should be associated with the observation as "device" participations."