201709 Implantables Tracking
Track Name
Point-of-care Medical Device and Implantable Tracking
Submitting WG/Project/Implementer Group
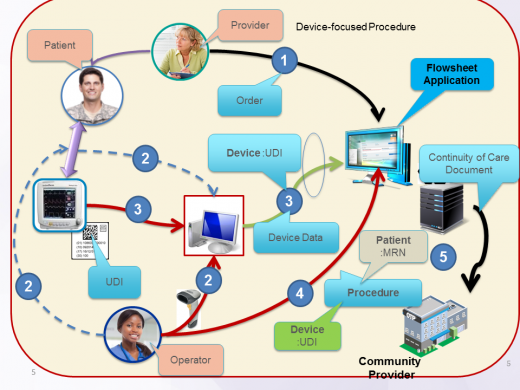
This track provides a set of simple test cases for Tracking Medical Devices (i.e. diagnostic, therapeutic, monitoring) and Implantables (biologics/tissue/cells and non-biologics/life-supporting/life-sustaining) at point of care addresses current problems relate to information accuracy and it provides procedure contextual information. This track specifies test cases related FHIR STU3 Procedure and Device resources used to report information from the point-of-care to enterprise system consistent with the IHE PCC Point-of-care Medical Device Tracking Supplement.
Justification
- Implantable medical devices are costly and concerns about illegitimate (i.e., counterfeit, stolen) products has become a global issue
- Post-market surveillance of implantable medical devices can be challenging
- Implantable medical device adverse events and recalls pose a patient safety issue
- Acquiring medical device data used at the point-of-care is difficult to retrieve for reuse at a later time
- Closes the loop on data acquisition at the point-of-care to support reporting of medical device data
- Medical device data used for:
- Continuum of care (e.g., Discharge Summary, Referrals)
- Registries (e.g., Total Joint Registry)
- Payers (e.g., government provided, private insurance)
- Can associate a medical device used for monitoring a disease or symptom of a disease (e.g., vital sign monitors, pulse oximeters, blood glucose monitors) to a patient for querying the device or procedure using the UDI
- Medical device data used for:
- Increase patient safety
- Traceability of medical devices (avoid use of counterfeit or illegitimate products)
- Quality issues identified (e.g., recalls, adverse events)
- Increase accurate medical device data capture at the point-of-care
- Eliminates human error from manual medical device data entry
Proposed Track Lead
Ioana Singureanu, VA/BZI, Skype: ioanasingureanu See Connectathon_Track_Lead_Responsibilities
Expected participants
- Department of Veterans Affairs
- Magpie360
- Eversolve, LLC
Roles
The test roles for this track are based on the IHE PCC PMDT Actors below:
Point-of-Care Medical Device Reporter
- Point-of-care client; it registers medical device, creates/updates point-of-care procedure information, query patient based on identifier scanned at the point-of-care. This role is played by a system used to track information about medical devices used at the point-of-care. The device and procedure information are populated at the point-of-care using scanned (AIDC) UDI and patient identifier to simplify the accurate tracking of this information.
Medical Device Server
- FHIR server, exposes Device, Procedure - references Patient. It is used to persist device and procedure information originating at the point of care. This information is made available to other information systems in the enterprise. This role may be played by a Medical Device Registry or an EHR system.
Point-of-Care Medical Device Requester
- This role is played by any information system that requires to compile an implantable device list for patient, evaluate outcomes based on device type (i.e. DI), respond manufacturers' recalls, and address patient safety events.
Scenarios
The following is a summary test cases proposed for
- Register (create) device record using
- UDI and DI only
- UDI and DI and optional attributes
- Start procedure...
- with externally referenced device
- Complete procedure
- with externally referenced device
- Complete implantable procedure
- with externally referenced device
- with contained device reference
- Search procedures
- by patient
- by other optional search parameters
- search devices
- by udi-carrier
- by patient
- by udi-di
Register Medical Device or Implantable from the point-of-care
- Action: The 'Tracker' creates a
Deviceresource (using POST/update), setsDevice.idto a 'GUID',Device.udi.carrierAIDCto the base64-encoded, 'scanned UDI', setsDevice.statusto 'active'. TheDevice.patientreferences is set to external reference previously retrievedPatientresource. (see 'Precondition'). - Precondition: The 'Tracker' queried the
Patientresource using thePatient.identifierscanned at the point-of-care. This external references is used inDevice.patient. - Success Criteria: The 'Requester' queries
Deviceby 'udi-carrier' (expression:Device.udi.carrierHRF | Device.udi.carrierAIDC) and retrieves one matching resource containing the correct information. The 'Tracker' can retrieve it by logical id/GUID. - Bonus point: specify optional data elements (S, 0..) of the
Deviceresource.
Complete an Implantable Procedure
- Action: The 'Tracker' creates a
Procedureresource (using POST/update), sets theProcedure.idto a GUID,Procedure.statusto 'completed',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedDateTimeto the current date/time. The 'Tracker' specifies theProcedure.codecorresponding the implant procedure performed. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Requester' searches the
Procedureresource based onProcedure.code(expression:code).
Start a Point-of-Care Procedure
- Action: The 'Tracker' creates a
Procedureresource (using POST/update), sets theProcedure.idto GUID,Procedure.statusto 'in-progress',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedPeriod.startto the current date/time. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Requester' searches the
Procedureresource based onProcedure.code(expression:code).
Complete an Implantable Procedure
- Action: The 'Tracker' updates the
Procedureresource (using POST/update), it sets theProcedure.idto its previously set GUID,Procedure.statusto 'completed',Procedure.focalDevice.manipulatedto reference the device registered above. The 'Tracker' set theProcedure.performedPeriod.endto the current date/time. The 'Tracker' specifies theProcedure.codecorresponding the implant procedure performed. - Precondition: The 'Tracker' queries the
Patientresource using thePatient.identifierscanned at the point-of-care. The 'Tracker' registered a medical device or implantable using a pre-defined GUID as its logical identifier. The 'Tracker' created a first version of theProcedure - Success Criteria: The tracker can retrieve the
Procedureresource using its _id. The 'Requester' queriesProcedurebased on the Patient reference. - Bonus point: The 'Requester' searches the
Procedureresource based onProcedure.code(expression:code).