ManufacturedItemDefinition FHIR Resource Proposal
Contents
- 1 MedicinalProductDefinition
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 RIM scope
- 1.7 Resource appropriateness
- 1.8 Expected implementations
- 1.9 Content sources
- 1.10 Example Scenarios
- 1.11 Resource Relationships
- 1.12 Timelines
- 1.13 gForge Users
- 1.14 When Resource Proposal Is Complete
- 1.15 FMG Notes
MedicinalProductDefinition
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019 (earlier approval as "RegulatedManufacturedItem" 13th September 2017)
Contributing or Reviewing Work Groups
- Pharmacy
- Orders and Observations
FHIR Resource Development Project Insight ID
1367
Scope of coverage
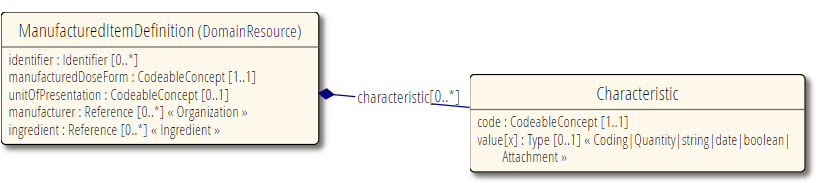
ManufacturedItemDefinition is to be used when you need to describe an actual physical medication item such as a tablet or capsule. This is typically for regulatory or manufacturing use cases.
This is the only resource that treats the "tablet" (or whatever it may be) as a physical object. It is still definitional however - it describes all instances of this tablet.
The resource aims to capture the definition of a single medication item type, such as a tablet of aspirin with a certain form, shape, manufacturer.
It is not intended to describe and be instantiated for each medication item, i.e., it’s not an actual real instance of a tablet, but rather represents e.g., all tablets of the same medication item type that are described by the ManufacturedItemDefinition.
To define several package variations, e.g. pack of 10 tablets and of 50 tablets, you would need only one ManufacturedItemDefinition, referenced from both (and associated with a numeric quantity of 10 or 50 in the respective PackagedProductDefintions).
To prescribe or dispense 100 tablets, you would not generally need this resource at all, because usually the code of the medication as a whole is sufficient, plus a quantity, e.g. 100 of "ABC123" (=aspirin 75 mg).
The definition needs to be a resource, rather than just a shared concept such as a data type, because the same physical item can appear in many different package types (which may come along after the item has been defined). (Gforge tracker item 23924 specifically requests this use case: "How to express multiple package configurations of the same manufactured item?")
This resource excludes devices and other items found in medication packaging such as scissors, bottle stoppers, applicators and so on (which would use DeviceDefinition).
This detail would very rarely be needed when prescribing – for which the correct resource is Medication. If you need to know what size, shape or colour the tablet is, or which packaging variations it can appear in, this resource has that level of detail.
The manufactured item describes the physical characteristics of the item, as opposed to its pharmaceutical aspects (which may only apply after mixing with other items - to become an AdministrableProductDefinition), or its packaging, or legal aspects (which apply only to the whole group of packing and manufactured items). This further differs from the administrable item in that the manufactured quantity may be different to the administered quantity, due to "overage" (manufacturing more in case some gets lost in the final preparation that the patient performs).
Note that there can be several types of manufactured item in one package (e.g. a combination pill product, or a cream and a tablet).
There are typically multiple instances of the item in the packaging and this is indicated by the quantity within the package (e.g. a package of 12 tablets), referencing a single copy of the manufactured item.
In scope:
Drug form and unit of presentation (e.g. solid, tablet, film-coated tablet).
Manufacturer and ingredients.
Physical characteristics, e.g. size, shape, color, image, imprints (logo/text)
Not in scope:
For day to day prescribing, the resource to use is Medication. This covers basic drug information – code, form, strength, batch number.
Devices - this does not cover devices, unless they have a pharmaceutical aspect, such as an impregnated bandage.
(Note that the lines between devices, biologicals and medicinal products are unclear in the real world, and this resource does not attempt to solve that issue by being prescriptive.)
Pricing and contextual or local formulary information - for that see MedicationKnowledge.
Some other closely related information is in scope of other proposed resources, but not in scope of this resource:
Detailed packaging information (PackagedProductDefinition) - this resource "contains" ManufacturedItemDefinitions
Pharmaceutical aspects of the product (AdminstrableProductDefinition) - can be created from one or more ManufacturedItemDefinitions (possibly mixed together)
Regulatory authorizations (RegulatedAuthorization) - legal aspects of the product as a whole (of which the manufactured item is a key component).
Details of ingredients of the manufactured item (Ingredient) and their substances (SubstanceDefinition).
RIM scope
Similar in scope to the product parts of CPM. Entity: Material (EntityClass="MAT") determinerCode=KIND
Resource appropriateness
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases.
This resource does not intend to clash with the existing Medication resource, but complements it with an extra level of detail. It is definitional rather than about an instance of a medication. (Also note however that when prescribing there is a duality of instance vs definition even in the Medication resource.)
This resource has been designed in close consultation with Pharmacy WG, and in conjunction with the MedicationKnowledge resource
Drug manufacturers currently submit this data electronically to regulators, when products are registered or altered, or marketing situations change.
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). They are required to move to IDMP, and this is a good opportunity to use a standards-based FHIR solution.
FDA for drug submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
Norwegian SAFEST project implementation https://github.com/HL7Norway/SAFEST
Content sources
A large amount of actual data of this kind exists in the EMA EU XEVMPD data base (and XEVPRM XML messages). Example FHIR data for several full product data sheets exists based on draft resources.
Also, information gained from early stage implementation of these resources at EMA (2018, 2019), and from many many received to EMA about the draft API specification from the European medicines regulatory network (https://www.ema.europa.eu/en/about-us/how-we-work/european-medicines-regulatory-network).
Also from FDA requirements (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments. Current active work on this project, with this resource.
This content is supported by the HL7 V3 SPL (Structured Product Labeling) standard.
Example Scenarios
Pharma companies submit details of new products to regulators. These include full product details down to the actual tablet level (also with packaging and other physical aspects).
Tablets or other physical representations can be stored and linked to multiple package variations, that may be created at later times.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators. This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). That scenario is currently being re-implemented, using this resource, as part of the EU wide SPOR project.
The Norwegian national project SAFEST, has this scenario:
The SAFEST project aims to deliver detailed packaging information in order to lay the groundwork for a “closed loop medication management”. A closed loop medication management requires bar codes on both the single dose administered by the patient and the outer packaging, in order to track the medicine from ordering, verifying, preparing to administering the medication.
The concept Packaged Medicinal Product holds information about each level of the package, including the place for the data carrier identifier for each level, and thus supports a closed loop medication management.
The concept Manufactured Item represent the medicinal product contained in the inner packaging, e.g «0,5 ml» in an ampoule with solution for injection. This information is also needed in the above mentioned closed loop and calculations needed for dosing of the medication. The amount of medicinal product, together with the strength gives how much of the active substance is given to the patient. When registered i electronic patient journal, the system can calculate based on this data. When the nurse is going to prepare an injection of 0,5 ml of a particular medicinal product, the system may show that an ampule from both the package with 3 ampoules of 0,5 ml and the package with 10 ampoules of 0,5 ml can be used.
Resource Relationships
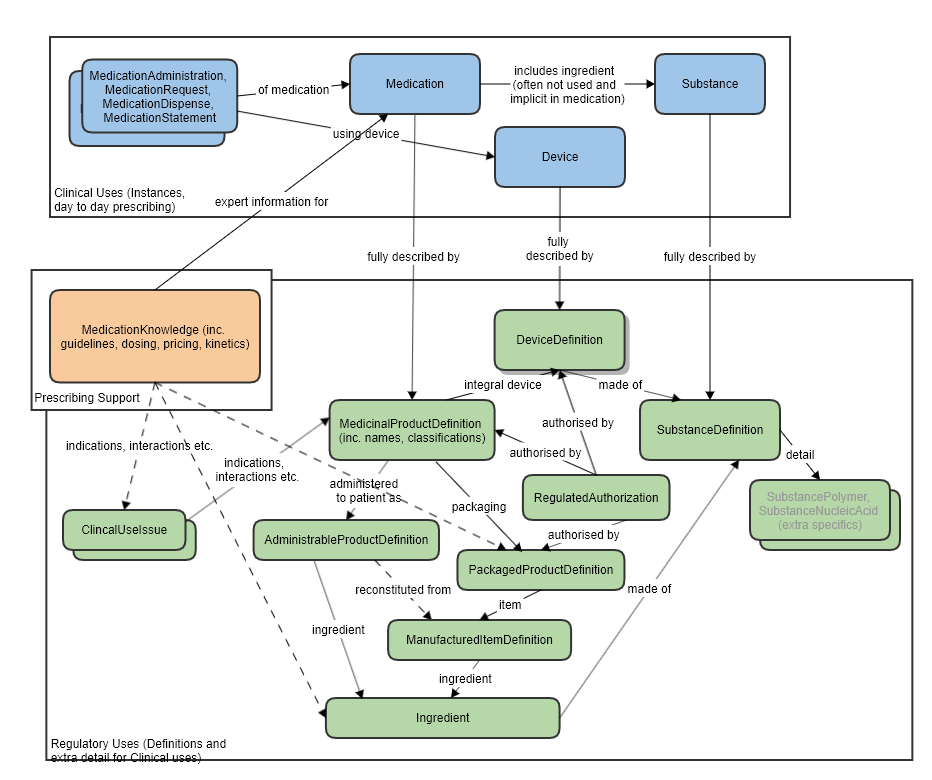
For the relationship to other resources, see the diagram below.
High level relationships of the main prescribing resources and the regulatory strata below:
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
The manufactured item is always physically within some packaging, so the PackagedProductDefinition resource has a reference to this item.
One or more manufactured items eventually forms the product that is directly administered to the patient, so the AdministrableProductDefinition also has a reference to this resource.
Some notable resource references:
Reference to Organization, for the manufacturer.
Reference to Ingredient for the ingredients of this manufactured item.
So:
MPD – as licenced
/ \
/ \____PPD1 or PPD2 – as supplied
/ / \
APD <-- MID1+MID2 (mixed)
\
as administered to the patient
MPD Medicinal Product (definition)
MID Manufactured Item (definition)
PPD PackagedProduct (definition)
APD Administrable Product (definition)
Timelines
Draft content is modelled in the FHIR build ManufacturedItemDefinition, with outline supporting documentation. Completion planned Q4 2019.
gForge Users
riksmithies
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org