RegulatedPackagedProduct FHIR Resource Proposal
Contents
- 1 RegulatedPackagedProduct
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 RIM scope
- 1.7 Resource appropriateness
- 1.8 Expected implementations
- 1.9 Content sources
- 1.10 Example Scenarios
- 1.11 Resource Relationships
- 1.12 Timelines
- 1.13 gForge Users
- 1.14 When Resource Proposal Is Complete
- 1.15 FMG Notes
RegulatedPackagedProduct
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019 (earlier approval as "MedicinalProductPackaged" 13th September 2017)
Contributing or Reviewing Work Groups
- Pharmacy
- Orders and Observations
- Clinical Decision Support
FHIR Resource Development Project Insight ID
1367
Scope of coverage
To support the content of the ISO 11615 IDMP Medicinal Product standard and other domain areas with similar requirements. 11615 covers detailed definition of products, their submissions to regulators, authorization activities, ingredients, packaging, accompanying devices, clinical particulars etc. Not all of those are expected to be covered in this single resource.
RIM scope
Similar in scope to the product parts of CPM. Entity: Material (EntityClass="MAT")
Resource appropriateness
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases.
This resource has been designed in close consultation with Pharmacy WG, and in conjunction with the MedicationKnowledge resource
RegulatedPackagedProduct is intended to add an extra level of product specification detail, such as is typically used by regulators, and only indirectly used during normal medication related work flows (e.g. for look-ups of unfamiliar products).
Drug manufacturers currently submit this data electronically to regulators, when products are registered or altered, or marketing situations change.
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). They are required to move to IDMP, and this is a good opportunity to use a standards-based FHIR solution.
FDA for drug submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
Content sources
The core basis for the resource is the information in ISO 11615 Medicinal Products standard, which is in turn partly based on the existing implementations in the EU and US. A large amount of actual data exists in the EMA EU XEVMPD data base (and XEVPRM XML messages). Example FHIR data for several full product data sheets exists based on draft resources.
Also, information gained from early stage implementation of these resources at EMA (2018, 2019), and from many many received to EMA about the draft API specification from the European medicines regulatory network (https://www.ema.europa.eu/en/about-us/how-we-work/european-medicines-regulatory-network).
Also from FDA requirements (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments.
Example Scenarios
Pharma companies submit details of new products to regulators, including the different packages that exist. The same "product" (drug, etc.) can be packaged in different way (20 tablet pack, 50 tablet pack etc), and may have different physical details and be licenced differently. Hence these are "addressable" entities, requiring a stand-alone resource.
Pharmacies and prescribers can view and download this information for reference and integration with their systems.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators. This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). That scenario is currently being re-implemented, using this resource, as part of the EU wide SPOR project.
Drug Manufacturing Quality information (aka PQ/CMC, Pharmaceutical Quality), as used by the FDA in the US. Specific plans to use this resource for that project.
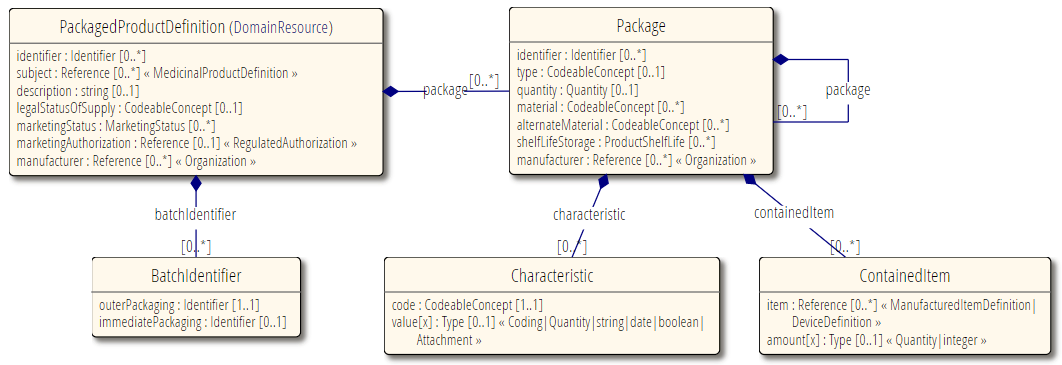
Resource Relationships
See diagram below.
Some notable resource references: Reference to Organization, for the manufacturer. Reference to RegulatedAuthorization for a pack specific legal authorization. Reference to directly supporting resources such as RegulatedMedicinalProduct, that this is a packing instance of (definitional instance).
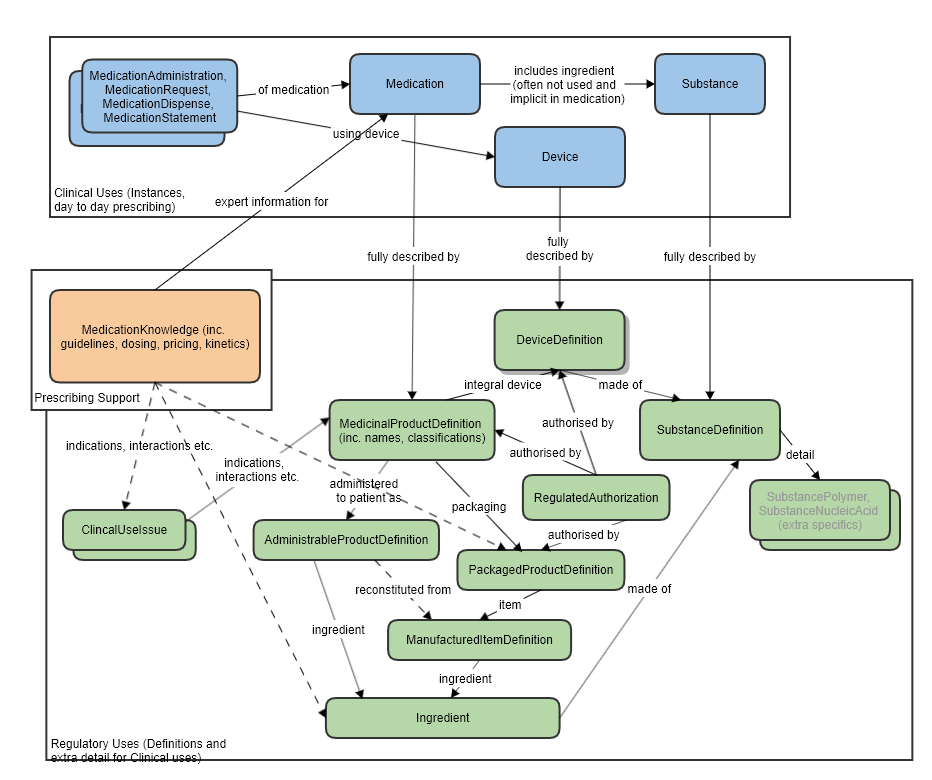
RegulatedPackagedProduct and Medication
This resource is intended to complement the Medication resource, which is focused on what is commonly needed for medical/clinical use cases. RegulatedPackagedProduct adds information needed for regulatory use cases, of which there is little overlap to day to day prescribing.
Most aspects of RegulatedPackagedProduct are not present in Medication at all, and are not current candidates for inclusion in the prescribe/dispense/administer workflow.
RegulatedPackagedProduct and MedicationKnowledge
MedicationKnowledge resource is aimed at drug knowledge bases. There is partial overlap in scope between that resource and some aspects of regulatory use cases. Where packaging information is needed, it is anticipated that this resource would be used. The boundaries between all these resource have been carefully thought out and have had much discussion in workgroups (BR&R, Pharmacy, CDS) and with FMG representatives.
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
High level relationships of the main prescribing resources and the regulatory strata below:
Timelines
Draft content is modelled in the FHIR build (http://build.fhir.org/regulatedmedicinalproduct.html), with outline supporting documentation. Completion planned Q4 2019.
gForge Users
riksmithies
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org