AdministrableProductDefinition FHIR Resource Proposal
Contents
- 1 AdministrableProductDefinition
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 Resource appropriateness
- 1.7 Expected implementations
- 1.8 Content sources
- 1.9 Example Scenarios
- 1.10 Resource Relationships
- 1.11 Timelines
- 1.12 gForge Users
- 1.13 When Resource Proposal Is Complete
- 1.14 FMG Notes
AdministrableProductDefinition
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019 (earlier approval as "MedicinalProductAuthorization" 13th September 2017)
Contributing or Reviewing Work Groups
- Pharmacy
FHIR Resource Development Project Insight ID
1367
Scope of coverage
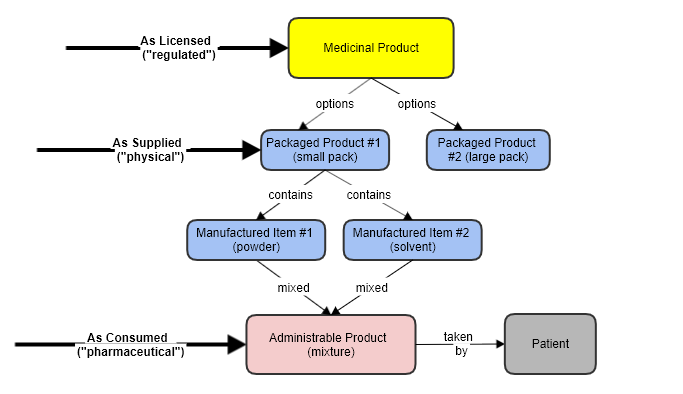
This resource covers the pharmaceutical as it is mixed, from the “manufactured” components in the pack, and as ready administered to the patient.
This is a different concept to the overall product (and to MedicinalProductDefinition specifically)
In effect this is a new substance, that doesn’t exist in the product otherwise. In some cases e.g. a tablet, the administrable form is the same as the manufactured form, but in the general case (e.g. a powder plus a solution for dissolving it) that is not true.
An Administrable Medicinal Product is a product that is ready to be administered to a patient. It may be created "on the fly" from several items of medication, not as ingredients, but as components that are combined for use.
This "product-mixed-for-use" is a very different thing to the components, or to package of all the components.
The Administrable item covers the drug as it is actually used. Only at this stage does it become a "pharmaceutical", and consequently it is only here that properties such Dose Form and Route Of Administration exist.
The relationship is this:
In scope:
Pharmaceutical aspects of the product. These don't exist in other resources.
Dose Form - as mixed, or "reconstituted"
Route of Administration - of the combined form
(Note that the overall product will also usually have a concatenated form "powder and solvent for injection")
Standard dosing information
Other coded pharmaceutical characteristics (e.g. rates of absorption etc).
Resource appropriateness
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases.
Regulators (EMA, FDA, and national "Competent Authorities"), as well as more local organisations, hospital and payer networks etc, need to record the legal authorizations that apply to medicinal products. This is a key focus of what organizations such as FDA and EMA do - they regulate and authorize the ability to make drugs available.
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). The EU wide system is currently being redeveloped to use FHIR, and this data is directly in scope.
FDA for Drug Submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
FDA for Pharmaceutical Quality (HL7 PSS approved, based on this resource, June 2019),
Content sources
The core basis for the resource is information currently used by FDA (as SPL) and the EMA EU XEVMPD data base (and XEVPRM XML messages).
Also, information gained from early stage implementation of this resources at EMA (2018, 2019), and from many many received to EMA about the draft API specification from the European medicines regulatory network (https://www.ema.europa.eu/en/about-us/how-we-work/european-medicines-regulatory-network).
Example Scenarios
Pharma companies submit details of new products to regulators, and include authorization details, which in turn may be updated by the regulators.
Pharmacies and prescribers can view and download this information for reference and integration with their systems. They may be able to see why a product was withdrawn for instance.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators.
This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). That scenario is currently being re-implemented, using this resource, as part of the EU wide SPOR project.
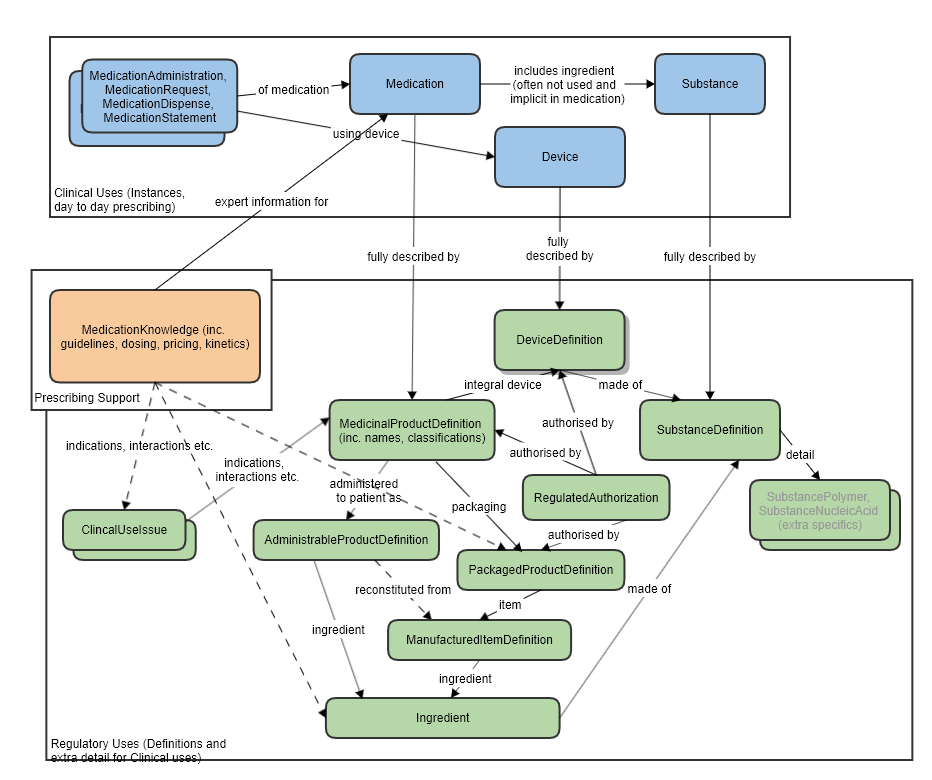
Resource Relationships
See diagram below.
Some notable resource references:
To Organization, for the licence holder and the regulator that established the authorization/licence.
To a MedicinalProductDefinition or PackagedProductDefinition (authorization can happen at different levels, per specific pack, or product wide), or a DeviceDefinition, as the subject, that is authorized.
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
High level relationships of the main prescribing resources and the regulatory strata below:
Timelines
Draft content is modelled in the FHIR build (http://build.fhir.org/regulatedauthorization.html), with outline supporting documentation. Completion planned Q4 2019.
gForge Users
riksmithies
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org