ClinicalUseIssue FHIR Resource Proposal
Contents
- 1 ClinicalUseIssue
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 RIM scope
- 1.7 Resource appropriateness
- 1.8 Expected implementations
- 1.9 Content sources
- 1.10 Example Scenarios
- 1.11 Resource Relationships
- 1.12 Timelines
- 1.13 gForge Users
- 1.14 When Resource Proposal Is Complete
- 1.15 FMG Notes
ClinicalUseIssue
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019
Contributing or Reviewing Work Groups
- Pharmacy
- Orders and Observations
- Clinical Decision Support
FHIR Resource Development Project Insight ID
1367
Scope of coverage
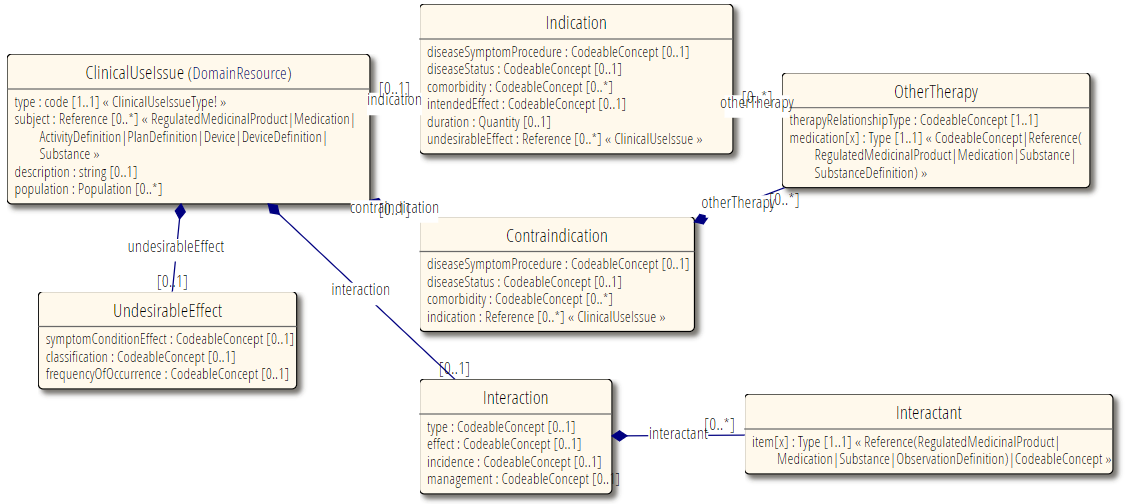
ClinicalUseIssue covers the background information about potential use of treatments or therapies (drugs, devices, procedures, substance etc). and how they affect a patient, positively and in particular negatively. The high level scope is:
indications - in what circumstances it is good practice to give the treatment
contraindications - in what circumstances it is NOT good practice to give the treatment
undesirable effects - what possible negative consequences are known to be issue for this treatment
interactions - what possible negative effects may be brought about by using this treatment (especially a drug), due to other previously employed drugs or treatments.
These are not related to any particular instance of use and also not any actual good or bad outcome, but are reference information, to guide the potential choice of treatments.
There is one resource for one set of facts about a single use pattern. This does not cover multiple indications, or both indications and contraindications in one.
It is a polymorphic resource, that can be one of several distinct things - an indication and interaction are very different things.
RIM scope
Similar in scope to the product parts of CPM. Entity: Material (EntityClass="MAT")
Resource appropriateness
This resource has been designed in consultation with CDS WG and Pharmacy WG, and in conjunction with the MedicationKnowledge resource
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). They are required to move to IDMP, and this is a good opportunity to use a standards-based FHIR solution.
FDA for drug submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
FDA for Pharmaceutical Quality (HL7 PSS approved, based on this resource, June 2019),
Content sources
The core basis for the resource is the information in ISO 11615 Medicinal Products standard, which is in turn partly based on the existing implementations in the EU and US. A large amount of actual data exists in the EMA EU XEVMPD data base (and XEVPRM XML messages). Example FHIR data for several full product data sheets exists based on draft resources.
Also, information gained from early stage implementation of these resources at EMA (2018, 2019), and from many many received to EMA about the draft API specification from the European medicines regulatory network (https://www.ema.europa.eu/en/about-us/how-we-work/european-medicines-regulatory-network).
Also from FDA requirements (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments.
Example Scenarios
Pharma companies submit details of new products to regulators. Updates are made when necessary e.g. clinical particulars change (a new contra-indication)
Pharmacies and prescribers can view and download this information for reference and integration with their systems.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators. This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). That scenarion is currently being re-implemented, using this resource, as part of the EU wide SPOR project.
Resource Relationships
See diagram below.
Some notable resource references: Reference to RegulatedPackagedProduct for the subject - the product that this is an indication for, for instance.
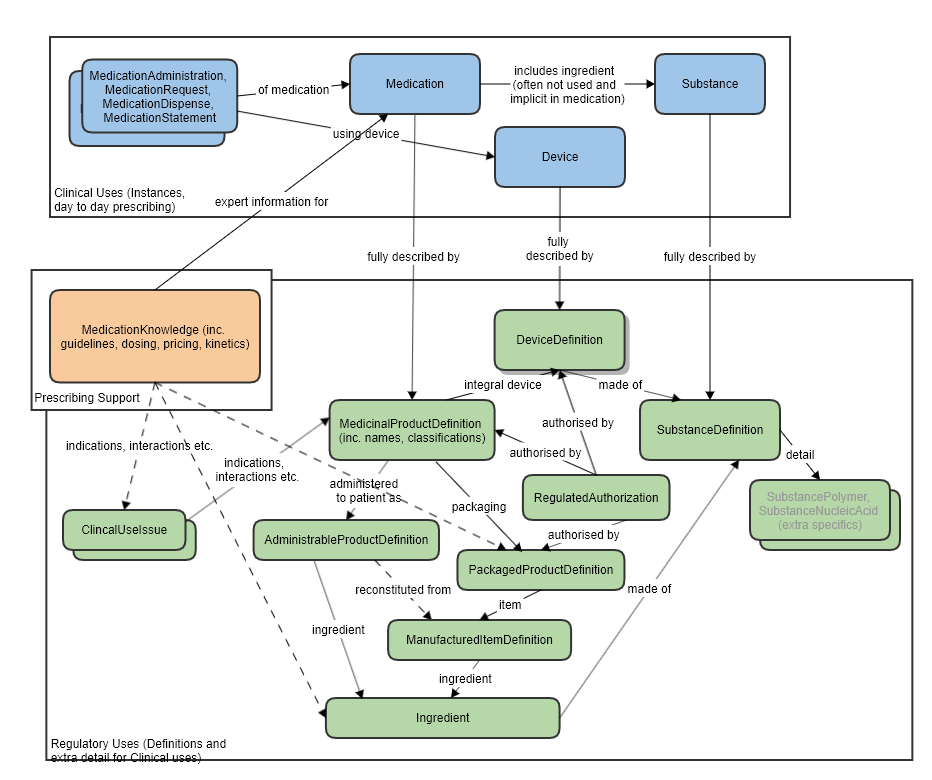
ClinicalUseIssue and MedicationKnowledge
MedicationKnowledge resource is aimed at drug knowledge bases. There is partial overlap in scope between that resource and some aspects of regulatory use cases. Where information about indications, contra-indications etc is needed, it is anticipated that this resource would be used.
The boundaries between all these resource have been carefully thought out and have had much discussion in workgroups (BR&R, Pharmacy, CDS) and with FMG representatives.
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
High level relationships of the main prescribing resources and the regulatory strata below:
Timelines
Draft content is modelled in the FHIR build (http://build.fhir.org/clinicaluseissue.html), with outline supporting documentation. Completion planned Q4 2019.
gForge Users
riksmithies
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org