Difference between revisions of "RegulatedMedicinalProduct FHIR Resource Proposal"
Riksmithies (talk | contribs) |
Riksmithies (talk | contribs) |
||
| Line 80: | Line 80: | ||
==Content sources== | ==Content sources== | ||
| − | The core basis for the resource is the information in ISO 11615 Medicinal Products standard. A large amount of actual data exists in the EMA XEVMPD data base and XEVPRM XML messages. Example FHIR data of several full product data sheets exists based on prototyped resources. | + | The core basis for the resource is the information in ISO 11615 Medicinal Products standard, which is in turn party based on the existing implementations in the EU and US. A large amount of actual data exists in the EMA EU XEVMPD data base and XEVPRM XML messages. Example FHIR data of several full product data sheets exists based on prototyped resources. |
| − | Also information gained from early stage implementation of these resources at EMA, and from FDA (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments. | + | Also, information gained from early stage implementation of these resources at EMA (2018, 2019), and from FDA (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments. |
==Example Scenarios== | ==Example Scenarios== | ||
Revision as of 08:50, 14 June 2019
Contents
- 1 RegulatedMedicinalProduct
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 RIM scope
- 1.7 Resource appropriateness
- 1.8 Expected implementations
- 1.9 Content sources
- 1.10 Example Scenarios
- 1.11 Resource Relationships
- 1.12 Timelines
- 1.13 gForge Users
- 1.14 When Resource Proposal Is Complete
- 1.15 FMG Notes
RegulatedMedicinalProduct
Owning work group name
Committee Approval Date:
6th May 2019 (earlier approval as "MedicinalProduct" 13th September 2017)
Contributing or Reviewing Work Groups
- Pharmacy
- Orders and Observations
- Clinical Decision Support
FHIR Resource Development Project Insight ID
1367
Scope of coverage
To support the content of the ISO 11615 IDMP Medicinal Product standard and other domain areas with similar requirements. 11615 covers detailed definition of products, their submissions to regulators, authorization activities, ingredients, packaging, accompanying devices, clinical particulars etc. Not all of those are expected to be covered in this single resource.
RIM scope
Similar in scope to the product parts of CPM. Entity: Material (EntityClass="MAT")
Resource appropriateness
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases.
This resource does not intend to clash with the existing Medication resource, but complements with an extra level of detail. It is seen as a sibling rather than a parent or a "superclass" to be profiled.
(The superclass option has widely discussed and rejected, since this would mean the Medication resource - by far the most commonly used - would become more complicated, being a very small profile of a very large model. We don't want to introduce such confusing complexity in that space - which is largely separate.)
This resource has been designed in close consultation with Pharmacy, and in conjunction with the MedicationKnowledge resource
It is intended to add an extra level of product specification detail, such as is typically used by regulators, and only indirectly used during normal medication related work flows (e.g. for lookups of unfamiliar products).
Drug manufacturers currently submit this data electronically to regulators, when products are registered or altered, or marketing situations change.
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). They are required to move to IDMP, and this is a good opportunity to use a standards-based FHIR solution.
FDA for drug submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
FDA for Pharmaceutical Quality (HL7 PSS approved, based on this resource, June 2019),
Content sources
The core basis for the resource is the information in ISO 11615 Medicinal Products standard, which is in turn party based on the existing implementations in the EU and US. A large amount of actual data exists in the EMA EU XEVMPD data base and XEVPRM XML messages. Example FHIR data of several full product data sheets exists based on prototyped resources.
Also, information gained from early stage implementation of these resources at EMA (2018, 2019), and from FDA (for PQ/CMC) and other workgroup review (BR&R, Pharmacy) and their comments.
Example Scenarios
Pharma companies submit details of new products to regulators. Updates are made when necessary e.g. Clinical particulars change (a new contra-indication), a new marketing authorization exists etc.
Pharmacies and prescribers can view and download this information for reference and integration with their systems.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators. This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). Currently being re-implmented with this resource as part of the EU wide SPOR project.
Drug Manufacturing Quality information (aka PQ/CMC, Pharmaceutical Quality), as used by the FDA in the US. Specific plans to use this resource for that project.
Resource Relationships
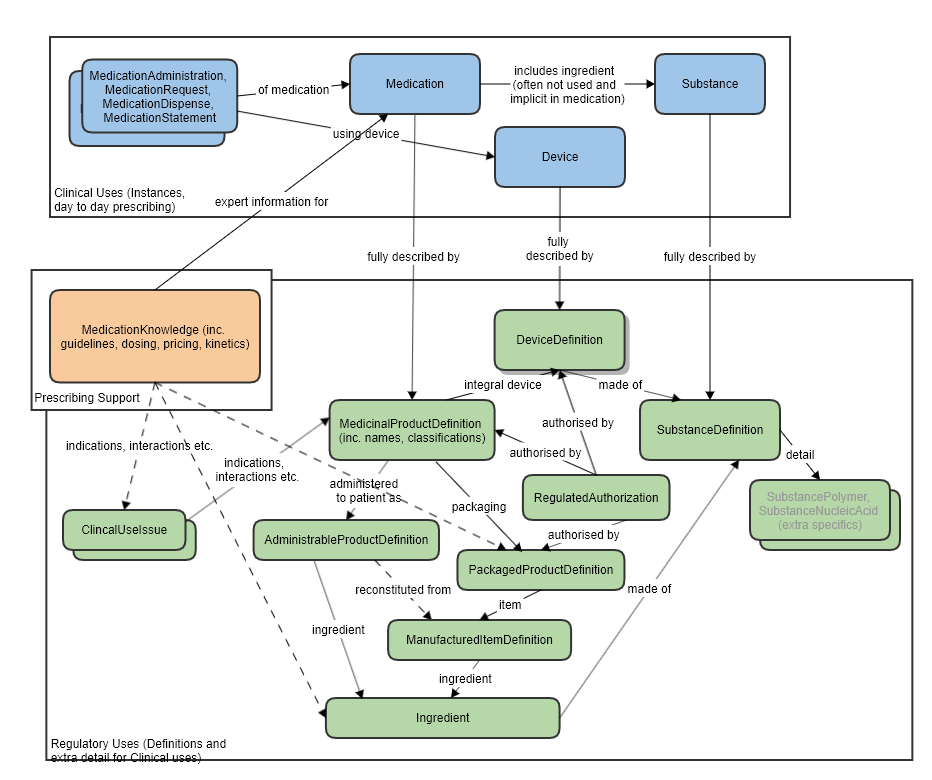
See diagram below.
Reference to Organization, for the manufacturer, regulator and other establishments. Reference to DocumentReference, for the regulatory submission documentation. Reference to (proposed) SubstanceSpecification to describe ingredients in detail. Reference to directly supporting (proposed) resources such as RegulatedAuthorization, RegulatedPackagedProduct (Indirect) reference to DeviceDefinition, via the other proposed resources (which was created with input from this IDMP project and includes our device requirements).
RegulatedMedicinalProduct and Medication
This resource is intended to complement the Medication resource, which is focused on what is commonly needed for medical/clinical use cases. RegulatedMedicinalProduct adds information needed for regulatory use cases, of which there is little overlap. MedicationKnowledge resource is aimed at drug knowledge bases . Where there is overlap in scope that and between regulatory use cases, the common associated resources of RegulatedMedicinalProduct will be used (e.g. Ingredient, ClinicalUseIssue). The boundaries between all these resource have been carefully thought out and have had much discussion in workgroups (BR&R, Pharmacy, CDS) and with FMG representatives.
Most aspects of RegulatedMedicinalProduct are not present in Medication at all, and are not current candidates for inclusion in the prescribe/dispense/administer workflow.
RegulatedMedicinalProduct and MedicationKnowledge
MedicationKnowledge resource is aimed at drug knowledge bases. Where there is overlap in scope that and between regulatory use cases, the common associated resources of RegulatedMedicinalProduct will be used (e.g. Ingredient, ClinicalUseIssue). The boundaries between all these resource have been carefully thought out and have had much discussion in workgroups (BR&R, Pharmacy, CDS) and with FMG representatives. Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
Timelines
Early draft by December 2017 comment-only ballot.
gForge Users
riksmithies (already has commit permission)
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org