Difference between revisions of "Conference Calls"
DarylThomas (talk | contribs) |
DarylThomas (talk | contribs) |
||
| Line 1: | Line 1: | ||
| + | ==WebEx Details== | ||
| + | * Go to https://dfci.webex.com/dfci/j.php?ED=142620047&UID=0&PW=NODc2OTNmMGQ1&RT=MiMxMQ%3D%3D | ||
| + | * Enter your name and email address. | ||
| + | * Enter the meeting password: genomics | ||
| + | * Click "Join Now". | ||
| + | * Telephone Number: 770-657-9270 Passcode: 941378 | ||
| + | ---- | ||
| + | ---- | ||
| + | |||
| + | =August 2, 2011= | ||
| + | ==Agenda== | ||
| + | * HL7 WGM September 2011 in San Diego: mini-symposium W Q1/Q2 to encourage community engagement | ||
| + | |||
| + | ==Attendees== | ||
| + | * Mollie Ullman-Cullere – Dana Farber | ||
| + | * Daryl Thomas – Life Technologies | ||
| + | * Don Rule – Translational Software | ||
| + | * Grant Wood - Intermountain Healthcare | ||
| + | * Lynn Bry - CAP & MGH | ||
| + | |||
| + | ==Draft Minutes== | ||
| + | * Grant discussed issues with securing a room for the event | ||
| + | * Lynn described four use cases and will circulate after CAP approval | ||
| + | * add more here ... | ||
| + | |||
| + | ==Actions== | ||
| + | * Daryl to circulate NIST workshop proposal | ||
| + | * Daryl to prepare list of institutions / contacts | ||
| + | * Don to prepare letter describing the event | ||
| + | ---- | ||
| + | |||
| + | =July 26, 2011= | ||
| + | ==Agenda== | ||
| + | * | ||
| + | |||
| + | ==Attendees== | ||
| + | * Mollie Ullman-Cullere – Dana Farber | ||
| + | * Clyde Ulmer – FDA | ||
| + | * Daryl Thomas – Life Technologies | ||
| + | * Don Rule – Translational Software | ||
| + | * Grant Wood - Intermountain Healthcare | ||
| + | * Mukesh Sharma - WUSTL | ||
| + | * Joyce Hernandez - Merck | ||
| + | |||
| + | ==Draft Minutes== | ||
| + | * Agreed to ballot GTR for Comment Only | ||
| + | * Mollie will incorporate feedback for V2 implementation guide for balloting and receive further feedback via email | ||
| + | * Joyce and Mukesh will be collaborating with NCI on a Domain Analysis Model for all ‘omics. We need to update the scope statement to reflect the presence of NCI. | ||
| + | * Joyce and Mukesh discussed the DAM | ||
| + | ** It is intended as a high level structure for all ‘omics | ||
| + | ** Meant to cover Research, Clinical Trials, and Healthcare domains | ||
| + | ** Aimed at unifying the nomenclature among domains to make data sharing easier | ||
| + | ** They will circulate an outline to recruit collaborators | ||
| + | ---- | ||
| + | |||
=July 19, 2011= | =July 19, 2011= | ||
==Agenda== | ==Agenda== | ||
* GTR ballot | * GTR ballot | ||
* Tumor profiling | * Tumor profiling | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
| Line 33: | Line 81: | ||
** Definitions for Genetic Testing Kits and Genetic Tests that include the registry where the test is defined and data from the manufacturer of the test. The genetic test definition includes the region of the genome that is covered by the test | ** Definitions for Genetic Testing Kits and Genetic Tests that include the registry where the test is defined and data from the manufacturer of the test. The genetic test definition includes the region of the genome that is covered by the test | ||
* Agreed that next week (7/26) we will complete discussion about updates to the V2 Implementation Guide and then Grant will begin the discussion of… | * Agreed that next week (7/26) we will complete discussion about updates to the V2 Implementation Guide and then Grant will begin the discussion of… | ||
| − | |||
---- | ---- | ||
| Line 39: | Line 86: | ||
==Agenda== | ==Agenda== | ||
* GTR ballot | * GTR ballot | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
| Line 64: | Line 104: | ||
** Create a summarized communication channel so that all members do not have to attend all meetings | ** Create a summarized communication channel so that all members do not have to attend all meetings | ||
** It was decided that next week’s meeting (7/19) will include a brief prioritization session and a full discussion of the Tumor Profiling work | ** It was decided that next week’s meeting (7/19) will include a brief prioritization session and a full discussion of the Tumor Profiling work | ||
| − | |||
| − | |||
---- | ---- | ||
| Line 71: | Line 109: | ||
==Agenda== | ==Agenda== | ||
* GTR ballot | * GTR ballot | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
| Line 83: | Line 114: | ||
==Draft Minutes== | ==Draft Minutes== | ||
* Discussed GTR harmonization with recommendations in the litrature. | * Discussed GTR harmonization with recommendations in the litrature. | ||
| − | |||
---- | ---- | ||
| Line 90: | Line 120: | ||
* GTR ballot: section codes and vocab binding | * GTR ballot: section codes and vocab binding | ||
* Review of Mollie's ballot comments | * Review of Mollie's ballot comments | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
* Phil Pochon, Don Rule, Scott Bolte, Grant Wood, Daryl Thomas, Amnon Shabo, Mollie Ullman, Dr. Christopher Burrow | * Phil Pochon, Don Rule, Scott Bolte, Grant Wood, Daryl Thomas, Amnon Shabo, Mollie Ullman, Dr. Christopher Burrow | ||
| + | |||
==Draft Minutes== | ==Draft Minutes== | ||
* BALLOT COMMENTS submitted by Mollie Ullman on the GTR | * BALLOT COMMENTS submitted by Mollie Ullman on the GTR | ||
| Line 106: | Line 130: | ||
**Should change mutations used in DCM example to pathogenic and benign mutations of the same gene listed in dbSNP and thereby change the internal lab mutation identifier to an external standard. (Note the internal identifier can be retained if generalized). | **Should change mutations used in DCM example to pathogenic and benign mutations of the same gene listed in dbSNP and thereby change the internal lab mutation identifier to an external standard. (Note the internal identifier can be retained if generalized). | ||
**Review of message is a bit difficult. Harmonization with recommended genetic report format may help; however, analysis should be preformed to ensure both narrative and machine readable logic is completely captured in each section. | **Review of message is a bit difficult. Harmonization with recommended genetic report format may help; however, analysis should be preformed to ensure both narrative and machine readable logic is completely captured in each section. | ||
| − | + | * Discussion of GTR organization following published recommendations on the formatting of genetic test reports. General consensus of the group was to create a table capturing recommended formats from each of the three references with CTR in a fourth column. | |
| − | * Discussion of GTR organization following published recommendations on the formatting of genetic test reports. General consensus of the group was to create a table capturing recommended formats from each of the three references with CTR in a | ||
**REFERENCES | **REFERENCES | ||
| − | ** | + | *** Gulley ML, Braziel RM, Halling KC, et al. Clinical laboratory reports in molecular pathology. Arch. Pathol. Lab. Med. 2007;131(6):852-863. |
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17550311 | Available at: http://www.ncbi.nlm.nih.gov/pubmed/17550311 | ||
| − | ** | + | *** Lubin IM, McGovern MM, Gibson Z, et al. Clinician perspectives about molecular genetic testing for heritable conditions and development of a clinician-friendly laboratory report. J Mol Diagn. 2009;11(2):162-171 |
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19197001 | Available at: http://www.ncbi.nlm.nih.gov/pubmed/19197001 | ||
| − | ** | + | *** Up-to-date guidelines from American College of Medical Genetics is available here (starting at section G17, page 29): http://www.acmg.net/StaticContent/SGs/Section_G_2010.pdf |
| − | |||
* Dr. Burrow emphasized the importance of using current published clinical recommendations, for formatting genetic test reports. In addition, discussion/analysis of how the GTR incorporates the Analytic Result vs. Clinical Interpretation (of the result) should be done. | * Dr. Burrow emphasized the importance of using current published clinical recommendations, for formatting genetic test reports. In addition, discussion/analysis of how the GTR incorporates the Analytic Result vs. Clinical Interpretation (of the result) should be done. | ||
---- | ---- | ||
| Line 122: | Line 144: | ||
* Future agenda items: Daryl Thomas presenting on clinical sequencing workflow, my interactions with NIST (6/14), and the FDA meeting (6/23) on next week's call (6.28). | * Future agenda items: Daryl Thomas presenting on clinical sequencing workflow, my interactions with NIST (6/14), and the FDA meeting (6/23) on next week's call (6.28). | ||
* Future agenda items: Continued work on GTR and review of Mollie's ballot comments | * Future agenda items: Continued work on GTR and review of Mollie's ballot comments | ||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
* Grant Wood, Clyder Ulmer, Amnon Shabo, Phil Pochon, and Mollie Ullman-Cullere | * Grant Wood, Clyder Ulmer, Amnon Shabo, Phil Pochon, and Mollie Ullman-Cullere | ||
| Line 129: | Line 149: | ||
* Discussion of family history and workflow from generalist to specialist, potential release 2, ISO ballot, CPP | * Discussion of family history and workflow from generalist to specialist, potential release 2, ISO ballot, CPP | ||
* Amnon is looking for feedback on GTR sections. - recommendation came for comparing recommendations to published recommendations for the field. | * Amnon is looking for feedback on GTR sections. - recommendation came for comparing recommendations to published recommendations for the field. | ||
| − | |||
| − | |||
---- | ---- | ||
| Line 139: | Line 157: | ||
* Start discussion of the DMP (Decision Making Practices)draft just posted to the list | * Start discussion of the DMP (Decision Making Practices)draft just posted to the list | ||
* Review CG morning slide for Orlando WGM (draft is at our web page inthe HL7 main site) | * Review CG morning slide for Orlando WGM (draft is at our web page inthe HL7 main site) | ||
| − | |||
| − | |||
| − | |||
| − | |||
---- | ---- | ||
| Line 149: | Line 163: | ||
* Review Mission and Charter | * Review Mission and Charter | ||
* Review Genetic Reporting changes if needed | * Review Genetic Reporting changes if needed | ||
| − | + | ---- | |
| − | |||
| − | |||
| − | |||
| − | - | ||
| − | |||
| − | |||
| − | |||
=March 29, 2011= | =March 29, 2011= | ||
==Agenda== | ==Agenda== | ||
* Meeting is cancelled | * Meeting is cancelled | ||
| + | ---- | ||
| + | |||
=March 15, 2011= | =March 15, 2011= | ||
==Agenda== | ==Agenda== | ||
* CDA IG for GTR ballot preparation from 11-1 US EST | * CDA IG for GTR ballot preparation from 11-1 US EST | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
---- | ---- | ||
| Line 193: | Line 183: | ||
** A valid sample with cytogenetics chromosome analysis report (CDA-GeneticTestingReport-Cytogenetics-Sample-v3) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm | ** A valid sample with cytogenetics chromosome analysis report (CDA-GeneticTestingReport-Cytogenetics-Sample-v3) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm | ||
** Note that the cytogenegtics sample is consistent with the latest GTR while the hearning loss sample is not (both sample are valid CDAs though) | ** Note that the cytogenegtics sample is consistent with the latest GTR while the hearning loss sample is not (both sample are valid CDAs though) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Attendees== | ==Attendees== | ||
| Line 228: | Line 209: | ||
http://www.hl7.org/Special/committees/clingenomics/overview.cfm | http://www.hl7.org/Special/committees/clingenomics/overview.cfm | ||
* Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement | * Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
---- | ---- | ||
| − | |||
| − | |||
=February 28, 2011 - special worksession at 11am= | =February 28, 2011 - special worksession at 11am= | ||
| − | + | Work Session is Canceled. Review of Genetic Test Report will take place during Tuesday's meeting. | |
| − | |||
---- | ---- | ||
| − | |||
=February 22, 2011= | =February 22, 2011= | ||
| Line 263: | Line 226: | ||
http://www.hl7.org/Special/committees/clingenomics/overview.cfm | http://www.hl7.org/Special/committees/clingenomics/overview.cfm | ||
* Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement | * Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
---- | ---- | ||
| Line 289: | Line 238: | ||
==Attendees== | ==Attendees== | ||
| − | |||
* Clyde Ulmer (US Food and Drug Administration) | * Clyde Ulmer (US Food and Drug Administration) | ||
* Don Rule (Translational Software) | * Don Rule (Translational Software) | ||
| Line 298: | Line 246: | ||
==Draft Minutes== | ==Draft Minutes== | ||
| − | |||
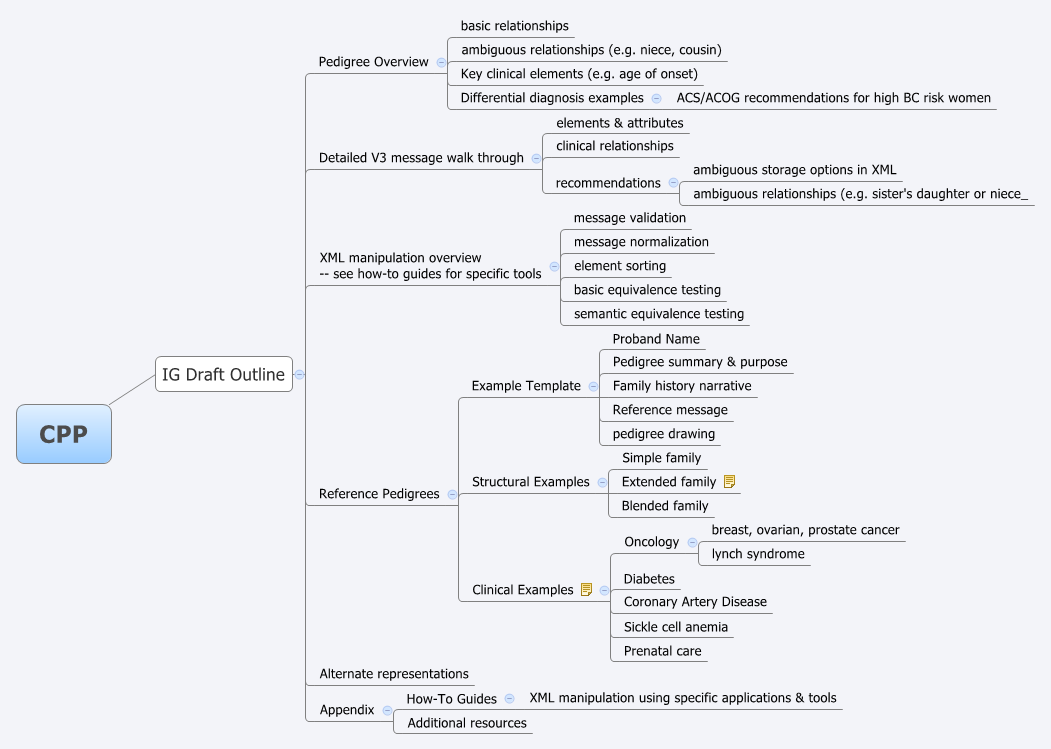
The entire call was dedicated to the introduction of the [[Canonical Pedigree Project | canonical pedigree project's]] outline for the implementation guide (see image below). | The entire call was dedicated to the introduction of the [[Canonical Pedigree Project | canonical pedigree project's]] outline for the implementation guide (see image below). | ||
| − | |||
* General feedback on the draft IG outline: | * General feedback on the draft IG outline: | ||
** Don: wants the CPP IG to include a high level list of message elements, relationships, and glossary. | ** Don: wants the CPP IG to include a high level list of message elements, relationships, and glossary. | ||
| Line 319: | Line 265: | ||
[[File:CPP-2011-02-15.png]] | [[File:CPP-2011-02-15.png]] | ||
| − | |||
---- | ---- | ||
| Line 345: | Line 290: | ||
**This is still a topic to be explored, as the group needs to better understand what the commitment is. Amnon and Yan will work on this. | **This is still a topic to be explored, as the group needs to better understand what the commitment is. Amnon and Yan will work on this. | ||
* DELAYED UNTIL APRIL (to enable focus on ballot work) - Review Work Group’s Decision Making Practices (DMP), Work Group’s Mission and Charter Statement (M&C), and Workgroup's location for posting minutes, decision making processes, and mission & charter statement | * DELAYED UNTIL APRIL (to enable focus on ballot work) - Review Work Group’s Decision Making Practices (DMP), Work Group’s Mission and Charter Statement (M&C), and Workgroup's location for posting minutes, decision making processes, and mission & charter statement | ||
| − | |||
---- | ---- | ||
Revision as of 01:13, 3 August 2011
Contents
- 1 WebEx Details
- 2 August 2, 2011
- 3 July 26, 2011
- 4 July 19, 2011
- 5 July 12, 2011
- 6 July 5, 2011
- 7 June 28, 2011
- 8 June 21, 2011
- 9 April 26, 2011
- 10 April 4, 2011
- 11 March 29, 2011
- 12 March 15, 2011
- 13 March 8, 2011
- 14 March 1, 2011
- 15 February 28, 2011 - special worksession at 11am
- 16 February 22, 2011
- 17 February 15, 2011
- 18 February 8, 2011
- 19 February 1, 2011
- 20 January 25, 2011
- 21 January 18, 2011
- 22 January 11, 2011
- 23 January 4, 2011
- 24 Archive - 2010 Conference Calls
- 25 November 2, 2010
- 26 November 9, 2010
- 27 November 16, 2010
- 28 November 23, 2010
- 29 November 30, 2010
- 30 December 7, 2010
- 31 December 14, 2010
- 32 December 21, 2010
WebEx Details
- Go to https://dfci.webex.com/dfci/j.php?ED=142620047&UID=0&PW=NODc2OTNmMGQ1&RT=MiMxMQ%3D%3D
- Enter your name and email address.
- Enter the meeting password: genomics
- Click "Join Now".
- Telephone Number: 770-657-9270 Passcode: 941378
August 2, 2011
Agenda
- HL7 WGM September 2011 in San Diego: mini-symposium W Q1/Q2 to encourage community engagement
Attendees
- Mollie Ullman-Cullere – Dana Farber
- Daryl Thomas – Life Technologies
- Don Rule – Translational Software
- Grant Wood - Intermountain Healthcare
- Lynn Bry - CAP & MGH
Draft Minutes
- Grant discussed issues with securing a room for the event
- Lynn described four use cases and will circulate after CAP approval
- add more here ...
Actions
- Daryl to circulate NIST workshop proposal
- Daryl to prepare list of institutions / contacts
- Don to prepare letter describing the event
July 26, 2011
Agenda
Attendees
- Mollie Ullman-Cullere – Dana Farber
- Clyde Ulmer – FDA
- Daryl Thomas – Life Technologies
- Don Rule – Translational Software
- Grant Wood - Intermountain Healthcare
- Mukesh Sharma - WUSTL
- Joyce Hernandez - Merck
Draft Minutes
- Agreed to ballot GTR for Comment Only
- Mollie will incorporate feedback for V2 implementation guide for balloting and receive further feedback via email
- Joyce and Mukesh will be collaborating with NCI on a Domain Analysis Model for all ‘omics. We need to update the scope statement to reflect the presence of NCI.
- Joyce and Mukesh discussed the DAM
- It is intended as a high level structure for all ‘omics
- Meant to cover Research, Clinical Trials, and Healthcare domains
- Aimed at unifying the nomenclature among domains to make data sharing easier
- They will circulate an outline to recruit collaborators
July 19, 2011
Agenda
- GTR ballot
- Tumor profiling
Attendees
- Amnon Shabo - IBM
- Mollie Ullman-Cullere – Dana Farber & Partners
- Clyde Ulmer – FDA
- Daryl Thomas – Life Technologies
- Don Rule – Translational Software
- Grant Wood - Intermountain Healthcare
- Mukesh Sharma - WUSTL
Draft Minutes
- Decision on how to proceed on the GTR – Amnon presented three options including i) proceed to Draft Standard for Trial Use (DSTU), ii) proceed for comments only, or iii) withdraw. The consensus was that we would like to move the specification forward and encourage further feedback but there is not enough time to obtain feedback and resolve outstanding issues. It was agreed to proceed for comments only with the document modified to clarify that it is not finalized, and to focus reviewers on specific parts of the document for which we need specific input.
- Motion to approve minutes passed with one minor update
- Review of modifications to Version 2 Implementation Guide to address tumor profiling including
- Incorporation of large genetic profiling result sets that do not include interpretation
- Inclusion of OMIM and COSMIC identifiers to provide further information about genetic mutations
- Linking references to PubMed, PharmGKB, or ClinicalTrials.gov
- Providing and Overall Interpretation for the results of a genetic test
- Updates to the LOINC answer lists based upon experience using the existing specification
- Extensions to the Genomic Source Class that are motivated by Tumor Profiling (e.g. a mutation may be labeled “likely somatic” if it is unlikely to be a germline mutation but no confirmatory test has been done)
- Addition of a Genetic Knowledge Reference Type
- Definitions for Genetic Testing Kits and Genetic Tests that include the registry where the test is defined and data from the manufacturer of the test. The genetic test definition includes the region of the genome that is covered by the test
- Agreed that next week (7/26) we will complete discussion about updates to the V2 Implementation Guide and then Grant will begin the discussion of…
July 12, 2011
Agenda
- GTR ballot
Attendees
- Amnon Shabo - IBM
- Mollie Ullman-Cullere – Dana Farber & Partners
- Clyde Ulmer – FDA
- Daryl Thomas – Life Technologies
- Don Rule – Translational Software
Draft Minutes
- A large part of the discussion was about what to ballot in September.
- There are mixed feelings about the Genetic Test Results (GTR) specification - on one hand it would be good to provide some guidance to the people that are actively developing solutions for exchanging genetic test results, on the other there is a concern that there has not been sufficient engagement on the clinical side to give it the rigor that is required, particularly for a clinical specification.
- Of particular concern is LOINC coding. The issue is that the coding affects the entire ecosystem including lab, physician, genetic counselor, and especially decision support systems. Further work needs to be done on the GTR so that it will work for clinical workflows. For instance, coded message structure should model practice standards for clinical reporting of genetics, if scope includes the clinical reporting.
- In addition concern was raised regarding ballot level of the current GTR document. The document is targeted at DSTU ballot level (as indicated in its project scope statement) but the final ballot level in each cycle is voted by the group prior to the submission deadline and the ballot level is corrected accordingly. It is important to note that the GTR passed DSTU ballot and in these cycles, with negatives which are still being addressed. we are reconciling negative comments and refining its structure and supplemental samples. Therefore, it is not yet in final DSTU status.
- There is a concern that some topics take unexpectedly long in working group discussions and that as a result we don’t get to all topics that are important (e.g. tumor profiling). A number of suggestions were made:
- Create parallel tracks that will discuss sub-topics and then coalesce into periodic meetings of the full working group
- Spend some time in each meeting discussing the logistics of the working group to strategize and prioritize the discussion
- Create a summarized communication channel so that all members do not have to attend all meetings
- It was decided that next week’s meeting (7/19) will include a brief prioritization session and a full discussion of the Tumor Profiling work
July 5, 2011
Agenda
- GTR ballot
Attendees
- Phil Pochon, Grant Wood, Amnon Shabo, Mollie Ullman, Clyde Ulmer
Draft Minutes
- Discussed GTR harmonization with recommendations in the litrature.
June 28, 2011
Agenda
- GTR ballot: section codes and vocab binding
- Review of Mollie's ballot comments
Attendees
- Phil Pochon, Don Rule, Scott Bolte, Grant Wood, Daryl Thomas, Amnon Shabo, Mollie Ullman, Dr. Christopher Burrow
Draft Minutes
- BALLOT COMMENTS submitted by Mollie Ullman on the GTR
- The importance of this guide to emerging standard for transmission and EHR documentation warrant the need to perform a GAP analysis, between recommended standards for report formatting (published in literature) and format of the GTR, and make revision accordingly. This analysis should be documented within the guide and references provided to the reader/implementer.
- Many systems will likely need to receive a mixture of messages; therefore, a GAP analysis between codes used in the v2 guides and GTR and make revisions accordingly. This analysis should be documented within the guide. Not all messages need to look exactly alike, but the elements do need to logically come together for patient care and discovery research. - Should change specific laboratories (e.g. HPCGG) to generic laboratories, if not a lab generated sample message.
- Should change mutations used in DCM example to pathogenic and benign mutations of the same gene listed in dbSNP and thereby change the internal lab mutation identifier to an external standard. (Note the internal identifier can be retained if generalized).
- Review of message is a bit difficult. Harmonization with recommended genetic report format may help; however, analysis should be preformed to ensure both narrative and machine readable logic is completely captured in each section.
* Discussion of GTR organization following published recommendations on the formatting of genetic test reports. General consensus of the group was to create a table capturing recommended formats from each of the three references with CTR in a fourth column.
- REFERENCES
- Gulley ML, Braziel RM, Halling KC, et al. Clinical laboratory reports in molecular pathology. Arch. Pathol. Lab. Med. 2007;131(6):852-863.
- REFERENCES
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17550311
- Lubin IM, McGovern MM, Gibson Z, et al. Clinician perspectives about molecular genetic testing for heritable conditions and development of a clinician-friendly laboratory report. J Mol Diagn. 2009;11(2):162-171
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19197001
- Up-to-date guidelines from American College of Medical Genetics is available here (starting at section G17, page 29): http://www.acmg.net/StaticContent/SGs/Section_G_2010.pdf
- Dr. Burrow emphasized the importance of using current published clinical recommendations, for formatting genetic test reports. In addition, discussion/analysis of how the GTR incorporates the Analytic Result vs. Clinical Interpretation (of the result) should be done.
June 21, 2011
Agenda
- Future agenda items: Daryl Thomas presenting on clinical sequencing workflow, my interactions with NIST (6/14), and the FDA meeting (6/23) on next week's call (6.28).
- Future agenda items: Continued work on GTR and review of Mollie's ballot comments
Attendees
- Grant Wood, Clyder Ulmer, Amnon Shabo, Phil Pochon, and Mollie Ullman-Cullere
Approved Minutes
- Discussion of family history and workflow from generalist to specialist, potential release 2, ISO ballot, CPP
- Amnon is looking for feedback on GTR sections. - recommendation came for comparing recommendations to published recommendations for the field.
April 26, 2011
Agenda
- Orlando WGM Agenda (see latest draft on our wiki)
- Sydney WGM Minutes (where is the latest draft?)
- Start discussion of the DMP (Decision Making Practices)draft just posted to the list
- Review CG morning slide for Orlando WGM (draft is at our web page inthe HL7 main site)
April 4, 2011
Agenda
- Review Mission and Charter
- Review Genetic Reporting changes if needed
March 29, 2011
Agenda
- Meeting is cancelled
March 15, 2011
Agenda
- CDA IG for GTR ballot preparation from 11-1 US EST
March 8, 2011
Agenda
- Review and approve minutes
- CDA IG for GTR:
- Ballot reconciliation (spreadsheets with disposition comments for discussion are available through the CG page in the HL7 main site at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- Latest GTR version (CDAR2_IG_GENTESTRPT_R1_O2_2011MAY - 8Mar2011) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- A valid sample with cytogenetics chromosome analysis report (CDA-GeneticTestingReport-Cytogenetics-Sample-v3) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- Note that the cytogenegtics sample is consistent with the latest GTR while the hearning loss sample is not (both sample are valid CDAs though)
Attendees
Don Rule, Joyce Hernandez, Grant Wood, Clyde Ulmer, Mollie Ullman, Amnon Shabo, Michael Miller, and Mukesh Sharma
Draft Minutes
- Future Meeting Schedule
- March:
- Mar 1 - GTR;
- Mar 8 - GTR;
- Mar 15 - GTR ballot preparation from 11-1 US EST;
- Mar 22 - Release 2 V2 Genetic Variation;
- Deadline - Mar 27 - Final Ballot submission
- April:
- Dicision Making, Mission & Charter etc... review
- March:
March 1, 2011
Agenda
- Review and approve minutes
- May WG - first joint with Anatomical Pathology set for Tuesday Q3
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
February 28, 2011 - special worksession at 11am
Work Session is Canceled. Review of Genetic Test Report will take place during Tuesday's meeting.
February 22, 2011
Agenda
- Review and approve minutes
- Main topic: Genetic Test Report
- May WG - first joint with Anatomical Pathology set for Tuesday Q3
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
February 15, 2011
Agenda
- Canonical Pedigree Project discussion
- Answer questions from new participants.
- Expand list of sample structural pedigrees -- the pedigrees that represent different family structures
- Expand list of sample clinical pedigrees -- matched pairs of pedigrees that illustrate gain or loss of predictive power based on clinical observations (e.g. age of onset of disease). See Clinical Power for an oncology example.
- Review list of desired technical capabilities (e.g. validate pedigree message, normalize XML, basic message equivalence testing, etc.)
- Note - GTR will be discussed on the Feb 22 call
Attendees
- Clyde Ulmer (US Food and Drug Administration)
- Don Rule (Translational Software)
- Michael Miller (Institute for Systems Biology)
- Mollie Ullman (Dana-Farber Cancer Institute)
- Scott Bolte (GE Healthcare) -- canonical pedigree project leader
- Vaughn Edelson (Genetic Alliance)
Draft Minutes
The entire call was dedicated to the introduction of the canonical pedigree project's outline for the implementation guide (see image below).
- General feedback on the draft IG outline:
- Don: wants the CPP IG to include a high level list of message elements, relationships, and glossary.
- Mollie: likes breast cancer as an example, but thinks it is too simple in the genetics and phenotypic paradigm. Wants to make sure a more complex disease, cardiomyopathy for example, is included. Treating that disease can entail a family history that spans four or five generations. (See Use of genetics in the clinical evaluation of cardiomyopathy. by DP Judge in JAMA.)
- Clyde: provided personal feedback that clinicians, for example an internal medicine doc that he knows well, stress it needs to be kept simple. Scott responded that there needs to be a clear separation where patients may enter the bulk of their information on their own so clinicians only need to clarify and confirm the most relevant elements.
- Alternate representations
- Question: Is the project intended to deal with native representation of family history in alternate formats (e.g. CCD) or just as embedded payloads? Answer: validating full fidelity, or loss of information, before & after encoding in alternate representations is definitely in scope.
- Australia is encoding family history in V2. Talk to Andrew McIntyre (Medical Objects) for additional details.
- XML:
- Don: expressed frustration that the high-level HL7 tools make it difficult to validate and manipulate XML documents. Would like the option of working purely with XSD (XML schema definitions).
- Michael: shared that work with MAGE and FuGE exposed the hazard of ambiguity as to where and how data is stored in an XML document. That led to implementation ambiguities.
- Project communications:
- Mollie reiterated that delivery of HL7 distribution lists experience significant delays for some readers. She suggested that active individuals be listed on the To line of messages to ensure prompt delivery.
- Scott proposed the convention that the subject line for project related mail start with CPP.
February 8, 2011
Agenda
- Review and approve minutes
- Main topic: review Cytogenetics
- check on CG vocabulary facilitator role
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
Attendees
Amnon Shabo, Grant Wood, Yan Heris, Mollie Ullman-Cullere, Scott Bolte, Joyce Hernandez, Clyde Ulmer, and Phil Pochon
Draft Minutes
- Review and approve minutes
- Main topic: review Cytogenetics
- Yan presented and will be sending codes out to the list
- check on CG vocabulary facilitator role
- Yan will be working on Cytogenetics and passing lessons learned to group for vocabular work.
- This is still a topic to be explored, as the group needs to better understand what the commitment is. Amnon and Yan will work on this.
- DELAYED UNTIL APRIL (to enable focus on ballot work) - Review Work Group’s Decision Making Practices (DMP), Work Group’s Mission and Charter Statement (M&C), and Workgroup's location for posting minutes, decision making processes, and mission & charter statement
February 1, 2011
Agenda
Agenda:
- Review previous meeting minutes
- Implications of CPP IG not being accepted as a public document
- Ballot planning, if needed
- Coordinate with main office for listing of references from main HL7 website to the wiki
- Issues/Hot Topics section on wiki
- Listing HIMSS CG Overview on wiki
Attendees
Amnon Shabo, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Scott Bolte, Daryl Thomas
Draft Minutes
- Approved previous meeting minutes
- Implications of CPP IG not being accepted as a public document: CG will continue to track and link to the CPP wiki will be created from the CG main page
- Coordinate with main office for listing of references from main HL7 website to the wiki: Amnon to follow-up
- Issues/Hot Topics section on wiki: hold off on creation of this content due to resource constraints
- Listing HIMSS CG Overview on wiki: this content will be posted
- Future Meeting Schedule
- February:
- Feb 1 - CPP (or fill-in) & more ballot planning;
- Feb 8 - Cytogenetics;
- Feb 15 - GTR, focus on comments and vocabulary;
- Feb 22 - CPP;
- Deadline - Feb 27 - Intent to Ballot
- March:
- Mar 1 - GTR;
- Mar 8 - TBD;
- Mar 15 - CPP;
- Mar 22 - TBD;
- Deadline - Mar 27 - Final Ballot submission
- April:
- Dicision Making, Mission & Charter etc... review
- February:
January 25, 2011
Agenda
Agenda:
- Review previous meeting minutes
- Report/feedback on previous meetings items
- Ballot planning
Attendees
Amnon Shabo, Clyde Ulmer, Don Rule, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Joyce Hernandez, Scott Bolte, Daryl Thomas
Draft Minutes
- Review previous meeting minutes - approved
- Report/feedback on previous meetings items
- CPP will use the existing clinical genomics listserv and is currently considering ballot content for May
- Project proposal exploring "How to better engage with academia" should be submitted by the end of week
- Note was made to consider barriers which may stem from HL7 general business model (fee for access to standards (e.g. 1,200$ membership for start-up))
- Discussed IHIC 2011 attendance
- Ballot planning
- Genetic Test Report (GTR), Cytogenetics and CPP is currently considering ballot content for May
- Gene Expression in Sept
- In 2 weeks reconciliation of GTR comments
- Next Release of Family History (further discussions regarding updates, ties to CPP, ANSI requirements)
- Draft plans for future calls leading up to ballot deadlines:
- February:
- Feb 1 - CPP (or fill-in) & more ballot planning;
- Feb 8 - Tentative - GTR, focus on comments and vocabulary;
- Feb 15 - Tentative - Cytogenetics;
- Feb 22 - CPP;
- Deadline - Feb 27 - Intent to Ballot
- March:
- Mar 1 - GTR;
- Mar 8 - TBD;
- Mar 15 - TBD;
- Mar 22 - TBD;
- Deadline - Mar 27 - Final Ballot submission
- February:
January 18, 2011
Agenda
Agenda:
- Meeting report from January Workgroup meeting
- Planning for May Workgroup meeting (e.g. Joints O&O/Lab ThurQ1 and CDS ThurQ3)
- Vote to create CPP listserv
Attendees
Amnon Shabo, Clyde Ulmer, Don Rule, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Mukesh Sharma, Scott Bolte
Approved Minutes
- Meeting report from January Workgroup meeting
- Pharmacy would like to see Clinical Genomic data within Clinical Statement (CS) - agreed and recommend Hans B. as CS contact
- Members of the CDS workgroup have developed a v2 Family History as part of the Australian HIT initiative. In discussions at WG meeting, it was determined that this would be good for CG to review and roll into CPP – CG WG agreed and Scott Bolte will track
- GELLO and other CDS work to review (see link below) – feedback on next steps from group - Discuss next weeks conference call
- O&O/Labs – E-Links is working on developing O/E for Genetic Tests. O&O advised double checking on their timeline to see if we should wait and evaluate for appropriateness as usage or extension in HL7 - Hans B. (O&O) to start email thread
- Education/Marketing sponsoring project on engagement with academia (e.g. starting an e-Journal) w/ CG as an interested party – Approved
- Planning for May Workgroup meeting (e.g. Joints O&O/Lab ThurQ1 and CDS ThurQ3) - Approved
- CG Open House: Tuesday Q4 - Approved
- Vote to create CPP listserv - Approved
- HL7 Family History v2 Australian implementation will dock into CG through the CPP project (additional content on the FH v2 to be distributed)
- Links to CDS presentations given at the Jan 2011 Workgroup meeting (feedback and recommendations to the CG WG can be discussed on the next call):
January 11, 2011
No conference call due to HL7 workgroup meeting.
January 4, 2011
Agenda
Agenda:
- Vote Grant Wood as Acting Co-chair for Sydney - vote to take place in first 15 min so Grant can catch his plane
- Canonical Pedigree Project: brief pro/con discussion of changing target realm from US to Universal.
- Review remote voting procedures for this cycles co-chair elections
- Review material for Sydney meeting
- Further discussion around how we might improve 2 way communication with the larger global community - projects, lessons learned, use case etc...
Attendees
Amnon Shabo Mollie Ullman-Cullere Grant Wood Scott Bolte Don Rule Joyce Hernandez
Draft Minutes
- Grant Wood was voted as Acting Co-chair for Sydney
- Canonical Pedigree Project: changing to universal realm
- Reviewed remote voting procedures for this cycles co-chair elections
- Reviewed material for Sydney meeting
- Further discussion around how we might improve 2 way communication with the larger global community - projects, lessons learned, use case etc... --> Discussion focused on education for the larger community and customer demand (for vendors).
Archive - 2010 Conference Calls
November 2, 2010
Agenda
- Continue ballot reconciliation of CDA GTR
Attendees
Amnon Shabo Clyde Ulmer Grant Wood Mukesh Sharma Scott Bolte Bruce Bray Sanghoon Lee Mollie Ullman-Cullere
Draft Minutes
- Amnon proposed a collaborative effort where we use our HL7 wiki area to routinely document the agendas, attendees and minutes of our conf. calls.
- CDA GTR Reconciliation
- VA commnets
- Needs a volunteer to study the new vocabulary spec and see how we can align our specs to it
- Need to decide on dynamic versus static binding
- if dynamic is preferred, we can also creats concept domains in HL7 that only point to possible value sets in controlled vbocabularies like LOINC
- Organizational issues of the GTR should be discussed with MDHT developers as well as with the Structured Documents Work Group
- Open issue: Add a constraint that if somatic then don't populate allelic state and if germline or prenatal then SHALL populate the allelic state; what about the sex chromosomes?
- VA commnets
Meeting minutes is a link to approved minutes in the HL7 main site.
November 9, 2010
Agenda
- This call was cancelled due to an "HL7 Version 3 Ambassador Webinar" held at the same time.
November 16, 2010
Agenda
- Review of Canonical Pedigree Project scope statement. Draft statement send to distribution list 2010-11-15 by Scott Bolte.
- Create a Family Health History (Pedigree) page on the Wiki designed for public consumption (non-HL7).
Attendees
Amnon Shabo (IBM) Grant Wood Clyde Ulmer Constanze Coon Daryl Thomas Don Rule Joyce Hernandez michael Mollie Mukesh Scott Bolte (GE)
Draft Minutes
Meeting mintues is a link to approved minutes in the HL7 main site.
November 23, 2010
Agenda for 11 AM Eastern Call
- NCI Presentation on Generic Assay Model.
- When: Tuesday, November 23, 2010 11:00 AM-12:00 PM (UTC-05:00) Eastern Time (US & Canada).
- Special call-in number and web-conference link:
Call in number: 888-520-2187 PC: 2434592 Centra: http://ncicb.centra.com Event ID: Generic Assay
Attendees
Draft Minutes
Agenda for 12 noon Eastern Call
- Finish CG DAM Reconcilliation: rows # 28, #33, #52, # 109 and #115
- Review and further develop HL7 Grant's Family Health History (Pedigree)for non-HL7 people wiki page
Attendees
Draft Minutes
Meeting mintues is a link to approved minutes in the HL7 main site.
November 30, 2010
Agenda
- Open Discussion - Next Generation Pedigree Model and View (Allen Hobbs)
- Topics (Amnon: the following topics are just brain storming on what's next for the Pedigree spec)
- Reconstructing a pedigree on the fly
- Consent management
- Keeping the results of previous pedigrees and its additional info like curation, risk assessment results
- Dynamic proband (remodeling)
- Utilizing the latest Clinical Statement
- Reconstruction by linking existing pedigrees (e.g., father and mother pedigrees of a proband)
- These pedigrees will represent data that the person is willing to share (consent is provided upfront but is still a challenge in this approach)
- Could we accommodate all types of data?
- e.g., "Strong history on paternal side for colon cancer"
- See the JAMIA paper "Evaluation of family history information within clinical documents and adequacy of HL7 clinical statement and clinical genomics family history models for its representation: a case report"
- Pedigree-based risk assessment in a CDA GTR (Genetic Testing Report)?
- Is 'risk assessment 'genetic testing' or interpretation of clinical and genetic data?
- Changes following comments from the ISO ballots
- Technical approaches to developing a cannoical Pedigre(Scott)
- Developing light Pedigree schemas so that compliant instances are also compliant with the full-blown schemas
- Non-technical content on the Pedigree standard (Grant)
- Reconstructing a pedigree on the fly
Attendees
Amnon Shabo (IBM) Grant Wood Michael Miller Mukesh Mollie Kevin Don Rule Jim Harrison Allen Hobbs Scott Bolte
Draft Minutes
Meeting mintues is a link to approved minutes in the HL7 main site.
December 7, 2010
Agenda
- Open Discussion - Suggestions for Sydney meeting: Input from newer and long-time WG members will be valuable for this planning.
Attendees
Joyce Hernandez Clyde Ulmer Grant Wood Scott Bolte Mollie Ullman-Cullere Don Rule
Draft Minutes
December 14, 2010
Agenda
- Review and Iteration on materials for Sydney meeting: Input from newer and long-time WG members will be valuable.
Attendees
Draft Minutes
December 21, 2010
Agenda
- Review and Iteration on materials for Sydney meeting: Input from newer and long-time WG members will be valuable.
- Vote Grant Wood as Acting Co-chair for Sydney meeting