This wiki has undergone a migration to Confluence found Here
Difference between revisions of "CSCR-072 Device Participation"
Jump to navigation
Jump to search
(Add CSCR-072) |
(Update CR) |
||
| Line 2: | Line 2: | ||
{|width=100% cellspacing=0 cellpadding=2 border=1 | {|width=100% cellspacing=0 cellpadding=2 border=1 | ||
|| '''Submitted by:''' Austin Kreisler | || '''Submitted by:''' Austin Kreisler | ||
| − | || '''Revision date:''' | + | || '''Revision date:''' 12/21/2007 |
|- | |- | ||
|| '''Submitted date:''' 12/17/2007 | || '''Submitted date:''' 12/17/2007 | ||
| Line 14: | Line 14: | ||
== Recommendation == | == Recommendation == | ||
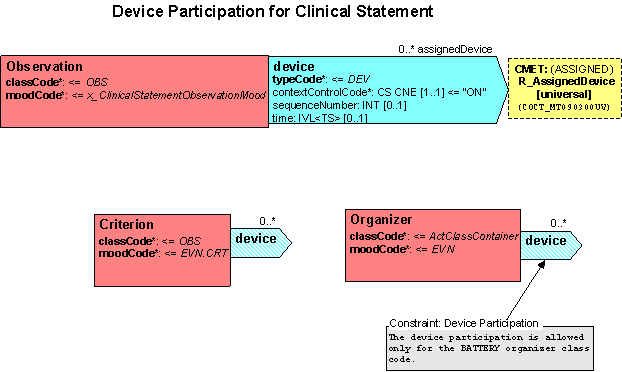
*Add a device (DEV) participation from Observation to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are: | *Add a device (DEV) participation from Observation to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are: | ||
| + | contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” | ||
| + | sequenceNumber (optional) INT [0..1] | ||
| + | time (optional) IVL<TS> [0..1] | ||
| + | *Add a device (DEV) participation from Organizer to the R_AssignedDevice universal CMET. Include a constraint indicating the participation is used only for the BATTERY organizer. Attributes to include on the DEV participation are: | ||
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” | contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” | ||
sequenceNumber (optional) INT [0..1] | sequenceNumber (optional) INT [0..1] | ||
| Line 28: | Line 32: | ||
According to the RIM definition for Observation.methodCode, documentation the test kits used should be handled as a device participation on the observation. From the RIM definition of Observation.methodCode: "...The methodCode should not be used to identify the specific device or test-kit material used in the observation. Such information about devices or test-kits should be associated with the observation as "device" participations." | According to the RIM definition for Observation.methodCode, documentation the test kits used should be handled as a device participation on the observation. From the RIM definition of Observation.methodCode: "...The methodCode should not be used to identify the specific device or test-kit material used in the observation. Such information about devices or test-kits should be associated with the observation as "device" participations." | ||
| − | [[Image:CSCR-072_DeviceParticipation.gif | + | [[Image:CSCR-072_DeviceParticipation.gif]] |
| + | |||
== Recommended Action Items == | == Recommended Action Items == | ||
== Resolution == | == Resolution == | ||
Latest revision as of 20:11, 21 December 2007
Back to Clinical Statement Change Requests page.
| Submitted by: Austin Kreisler | Revision date: 12/21/2007 |
| Submitted date: 12/17/2007 | Change request ID: CSCR-072 |
Issue
For some laboratory testing we have identified a requirement to communicate what testing kit was used to create a laboratory observation. There can also be test kit specific interpretation ranges associated with laboratory results.
Recommendation
- Add a device (DEV) participation from Observation to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are:
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” sequenceNumber (optional) INT [0..1] time (optional) IVL<TS> [0..1]
- Add a device (DEV) participation from Organizer to the R_AssignedDevice universal CMET. Include a constraint indicating the participation is used only for the BATTERY organizer. Attributes to include on the DEV participation are:
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” sequenceNumber (optional) INT [0..1] time (optional) IVL<TS> [0..1]
- Add a device (DEV) participation from Criterion to the R_AssignedDevice universal CMET. Attributes to include on the DEV participation are:
contextControlCode (required) CS CNE [1..1] ContextControl, default = “ON” sequenceNumber (optional) INT [0..1] time (optional) IVL<TS> [0..1]
Rationale
Discussion
According to the RIM definition for Observation.methodCode, documentation the test kits used should be handled as a device participation on the observation. From the RIM definition of Observation.methodCode: "...The methodCode should not be used to identify the specific device or test-kit material used in the observation. Such information about devices or test-kits should be associated with the observation as "device" participations."