CG WG Call Notes leading to 2011 May WGM
Back to Agendas and Minutes.
Contents
April 26, 2011
Agenda
- Orlando WGM Agenda (see latest draft on our wiki)
- Sydney WGM Minutes (where is the latest draft?)
- Start discussion of the DMP (Decision Making Practices)draft just posted to the list
- Review CG morning slide for Orlando WGM (draft is at our web page inthe HL7 main site)
April 4, 2011
Agenda
- Review Mission and Charter
- Review Genetic Reporting changes if needed
March 29, 2011
Agenda
- Meeting is cancelled
March 15, 2011
Agenda
- CDA IG for GTR ballot preparation from 11-1 US EST
March 8, 2011
Agenda
- Review and approve minutes
- CDA IG for GTR:
- Ballot reconciliation (spreadsheets with disposition comments for discussion are available through the CG page in the HL7 main site at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- Latest GTR version (CDAR2_IG_GENTESTRPT_R1_O2_2011MAY - 8Mar2011) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- A valid sample with cytogenetics chromosome analysis report (CDA-GeneticTestingReport-Cytogenetics-Sample-v3) is available at http://www.hl7.org/Special/committees/clingenomics/index.cfm
- Note that the cytogenegtics sample is consistent with the latest GTR while the hearning loss sample is not (both sample are valid CDAs though)
Attendees
Don Rule, Joyce Hernandez, Grant Wood, Clyde Ulmer, Mollie Ullman, Amnon Shabo, Michael Miller, and Mukesh Sharma
Draft Minutes
- Future Meeting Schedule
- March:
- Mar 1 - GTR;
- Mar 8 - GTR;
- Mar 15 - GTR ballot preparation from 11-1 US EST;
- Mar 22 - Release 2 V2 Genetic Variation;
- Deadline - Mar 27 - Final Ballot submission
- April:
- Decision Making, Mission & Charter etc... review
- March:
March 1, 2011
Agenda
- Review and approve minutes
- May WG - first joint with Anatomical Pathology set for Tuesday Q3
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
=February 28, 2011= - special worksession at 11am Work Session is Canceled. Review of Genetic Test Report will take place during Tuesday's meeting.
February 22, 2011
Agenda
- Review and approve minutes
- Main topic: Genetic Test Report
- May WG - first joint with Anatomical Pathology set for Tuesday Q3
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
February 15, 2011
Agenda
- Canonical Pedigree Project discussion
- Answer questions from new participants.
- Expand list of sample structural pedigrees -- the pedigrees that represent different family structures
- Expand list of sample clinical pedigrees -- matched pairs of pedigrees that illustrate gain or loss of predictive power based on clinical observations (e.g. age of onset of disease). See Clinical Power for an oncology example.
- Review list of desired technical capabilities (e.g. validate pedigree message, normalize XML, basic message equivalence testing, etc.)
- Note - GTR will be discussed on the Feb 22 call
Attendees
- Clyde Ulmer (US Food and Drug Administration)
- Don Rule (Translational Software)
- Michael Miller (Institute for Systems Biology)
- Mollie Ullman (Dana-Farber Cancer Institute)
- Scott Bolte (GE Healthcare) -- canonical pedigree project leader
- Vaughn Edelson (Genetic Alliance)
Draft Minutes
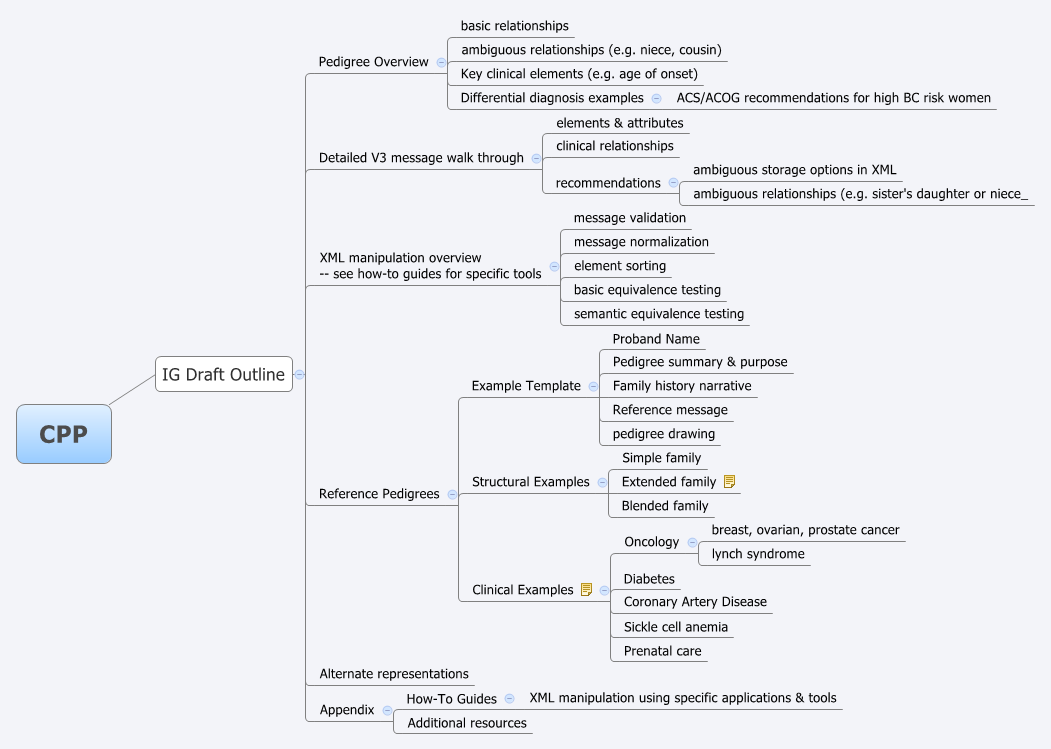
The entire call was dedicated to the introduction of the canonical pedigree project's outline for the implementation guide (see image below).
- General feedback on the draft IG outline:
- Don: wants the CPP IG to include a high level list of message elements, relationships, and glossary.
- Mollie: likes breast cancer as an example, but thinks it is too simple in the genetics and phenotypic paradigm. Wants to make sure a more complex disease, cardiomyopathy for example, is included. Treating that disease can entail a family history that spans four or five generations. (See Use of genetics in the clinical evaluation of cardiomyopathy. by DP Judge in JAMA.)
- Clyde: provided personal feedback that clinicians, for example an internal medicine doc that he knows well, stress it needs to be kept simple. Scott responded that there needs to be a clear separation where patients may enter the bulk of their information on their own so clinicians only need to clarify and confirm the most relevant elements.
- Alternate representations
- Question: Is the project intended to deal with native representation of family history in alternate formats (e.g. CCD) or just as embedded payloads? Answer: validating full fidelity, or loss of information, before & after encoding in alternate representations is definitely in scope.
- Australia is encoding family history in V2. Talk to Andrew McIntyre (Medical Objects) for additional details.
- XML:
- Don: expressed frustration that the high-level HL7 tools make it difficult to validate and manipulate XML documents. Would like the option of working purely with XSD (XML schema definitions).
- Michael: shared that work with MAGE and FuGE exposed the hazard of ambiguity as to where and how data is stored in an XML document. That led to implementation ambiguities.
- Project communications:
- Mollie reiterated that delivery of HL7 distribution lists experience significant delays for some readers. She suggested that active individuals be listed on the To line of messages to ensure prompt delivery.
- Scott proposed the convention that the subject line for project related mail start with CPP.
February 8, 2011
Agenda
- Review and approve minutes
- Main topic: review Cytogenetics
- check on CG vocabulary facilitator role
- Review Work Group’s Decision Making Practices (DMP)
URL: http://www.hl7.org/participate/decisionmaking.cfm
- review your Work Group’s Mission and Charter Statement (M&C)
Ours was last reviewed in 2009. These must be reviewed within two-year cycles.
http://www.hl7.org/Special/committees/clingenomics/overview.cfm
- Review Workgroup's location for posting minutes, decision making processes, and mission & charter statement
Attendees
Amnon Shabo, Grant Wood, Yan Heris, Mollie Ullman-Cullere, Scott Bolte, Joyce Hernandez, Clyde Ulmer, and Phil Pochon
Draft Minutes
- Review and approve minutes
- Main topic: review Cytogenetics
- Yan presented and will be sending codes out to the list
- check on CG vocabulary facilitator role
- Yan will be working on Cytogenetics and passing lessons learned to group for vocabular work.
- This is still a topic to be explored, as the group needs to better understand what the commitment is. Amnon and Yan will work on this.
- DELAYED UNTIL APRIL (to enable focus on ballot work) - Review Work Group’s Decision Making Practices (DMP), Work Group’s Mission and Charter Statement (M&C), and Workgroup's location for posting minutes, decision making processes, and mission & charter statement
February 1, 2011
Agenda
Agenda:
- Review previous meeting minutes
- Implications of CPP IG not being accepted as a public document
- Ballot planning, if needed
- Coordinate with main office for listing of references from main HL7 website to the wiki
- Issues/Hot Topics section on wiki
- Listing HIMSS CG Overview on wiki
Attendees
Amnon Shabo, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Scott Bolte, Daryl Thomas
Draft Minutes
- Approved previous meeting minutes
- Implications of CPP IG not being accepted as a public document: CG will continue to track and link to the CPP wiki will be created from the CG main page
- Coordinate with main office for listing of references from main HL7 website to the wiki: Amnon to follow-up
- Issues/Hot Topics section on wiki: hold off on creation of this content due to resource constraints
- Listing HIMSS CG Overview on wiki: this content will be posted
- Future Meeting Schedule
- February:
- Feb 1 - CPP (or fill-in) & more ballot planning;
- Feb 8 - Cytogenetics;
- Feb 15 - GTR, focus on comments and vocabulary;
- Feb 22 - CPP;
- Deadline - Feb 27 - Intent to Ballot
- March:
- Mar 1 - GTR;
- Mar 8 - TBD;
- Mar 15 - CPP;
- Mar 22 - TBD;
- Deadline - Mar 27 - Final Ballot submission
- April:
- Dicision Making, Mission & Charter etc... review
- February:
January 25, 2011
Agenda
Agenda:
- Review previous meeting minutes
- Report/feedback on previous meetings items
- Ballot planning
Attendees
Amnon Shabo, Clyde Ulmer, Don Rule, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Joyce Hernandez, Scott Bolte, Daryl Thomas
Draft Minutes
- Review previous meeting minutes - approved
- Report/feedback on previous meetings items
- CPP will use the existing clinical genomics listserv and is currently considering ballot content for May
- Project proposal exploring "How to better engage with academia" should be submitted by the end of week
- Note was made to consider barriers which may stem from HL7 general business model (fee for access to standards (e.g. 1,200$ membership for start-up))
- Discussed IHIC 2011 attendance
- Ballot planning
- Genetic Test Report (GTR), Cytogenetics and CPP is currently considering ballot content for May
- Gene Expression in Sept
- In 2 weeks reconciliation of GTR comments
- Next Release of Family History (further discussions regarding updates, ties to CPP, ANSI requirements)
- Draft plans for future calls leading up to ballot deadlines:
- February:
- Feb 1 - CPP (or fill-in) & more ballot planning;
- Feb 8 - Tentative - GTR, focus on comments and vocabulary;
- Feb 15 - Tentative - Cytogenetics;
- Feb 22 - CPP;
- Deadline - Feb 27 - Intent to Ballot
- March:
- Mar 1 - GTR;
- Mar 8 - TBD;
- Mar 15 - TBD;
- Mar 22 - TBD;
- Deadline - Mar 27 - Final Ballot submission
- February:
January 18, 2011
Agenda
Agenda:
- Meeting report from January Workgroup meeting
- Planning for May Workgroup meeting (e.g. Joints O&O/Lab ThurQ1 and CDS ThurQ3)
- Vote to create CPP listserv
Attendees
Amnon Shabo, Clyde Ulmer, Don Rule, Grant Wood, Jim Harrison, Michael Miller, Mollie Ullman-Cullere, Mukesh Sharma, Scott Bolte
Approved Minutes
- Meeting report from January Workgroup meeting
- Pharmacy would like to see Clinical Genomic data within Clinical Statement (CS) - agreed and recommend Hans B. as CS contact
- Members of the CDS workgroup have developed a v2 Family History as part of the Australian HIT initiative. In discussions at WG meeting, it was determined that this would be good for CG to review and roll into CPP – CG WG agreed and Scott Bolte will track
- GELLO and other CDS work to review (see link below) – feedback on next steps from group - Discuss next weeks conference call
- O&O/Labs – E-Links is working on developing O/E for Genetic Tests. O&O advised double checking on their timeline to see if we should wait and evaluate for appropriateness as usage or extension in HL7 - Hans B. (O&O) to start email thread
- Education/Marketing sponsoring project on engagement with academia (e.g. starting an e-Journal) w/ CG as an interested party – Approved
- Planning for May Workgroup meeting (e.g. Joints O&O/Lab ThurQ1 and CDS ThurQ3) - Approved
- CG Open House: Tuesday Q4 - Approved
- Vote to create CPP listserv - Approved
- HL7 Family History v2 Australian implementation will dock into CG through the CPP project (additional content on the FH v2 to be distributed)
- Links to CDS presentations given at the Jan 2011 Workgroup meeting (feedback and recommendations to the CG WG can be discussed on the next call):