Difference between revisions of "Ingredient FHIR Resource Proposal"
Riksmithies (talk | contribs) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<div style="float: left;">[[Image:OpenHotTopic.GIF|35px| ]]</div> | <div style="float: left;">[[Image:OpenHotTopic.GIF|35px| ]]</div> | ||
<div style="background:#F0F0F0"> | <div style="background:#F0F0F0"> | ||
| − | This page documents a [[:category: | + | This page documents a [[:category:Approved FHIR Resource Proposal|Approved ]] [[:category:FHIR Resource Proposal|FHIR Resource Proposal]] |
</div> | </div> | ||
</div> | </div> | ||
[[Category:FHIR Resource Proposal]] | [[Category:FHIR Resource Proposal]] | ||
| − | [[Category: | + | [[Category:Approved FHIR Resource Proposal]] |
| Line 56: | Line 56: | ||
This includes: | This includes: | ||
| − | Substance | + | Substance (including as one or more "specified" substance aspects including the grade or process) |
Strength expressed in different possible ways (presentation, concentration) and details of how that strength it is measured. | Strength expressed in different possible ways (presentation, concentration) and details of how that strength it is measured. | ||
Strength expressed as a function of a second substance (reference strength substance), for example as equivalent of a certain amount of the "salt". | Strength expressed as a function of a second substance (reference strength substance), for example as equivalent of a certain amount of the "salt". | ||
| + | |||
| + | Manufacturer. | ||
==Resource appropriateness== | ==Resource appropriateness== | ||
| Line 66: | Line 68: | ||
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases. | There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases. | ||
| − | + | This resource does not intend to clash with the existing Medication resource, but complements it with an extra level of detail. | |
| − | + | ||
| + | Drug manufacturers currently submit this data electronically to regulators, when products are registered or altered, or marketing situations change. | ||
| + | This resource has been designed in close consultation with Pharmacy WG - Medication knowledge bases also cover this level of detail and this resource is currently referenced within the MedicationKnowledge resource | ||
==Expected implementations== | ==Expected implementations== | ||
| Line 74: | Line 78: | ||
FDA for Drug Submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR). | FDA for Drug Submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR). | ||
| + | |||
| + | FDA for Pharmaceutical Quality (HL7 PSS approved, based on this resource, June 2019). | ||
==Content sources== | ==Content sources== | ||
| Line 83: | Line 89: | ||
==Example Scenarios== | ==Example Scenarios== | ||
| − | Pharma companies submit details of new products to regulators, and include | + | Pharma companies submit details of new products to regulators, and include ingredient details. |
| + | |||
| + | Pharmacies and prescribers can view and download this information for reference and integration with their systems. | ||
| − | + | Decision support systems can use ingredient substance information for allergy checking. | |
Specific use cases include: | Specific use cases include: | ||
| Line 99: | Line 107: | ||
Some notable resource references: | Some notable resource references: | ||
| − | To a | + | To a manufacturing organization. Incoming relationships from the pharmaceutical products that use the ingredient. |
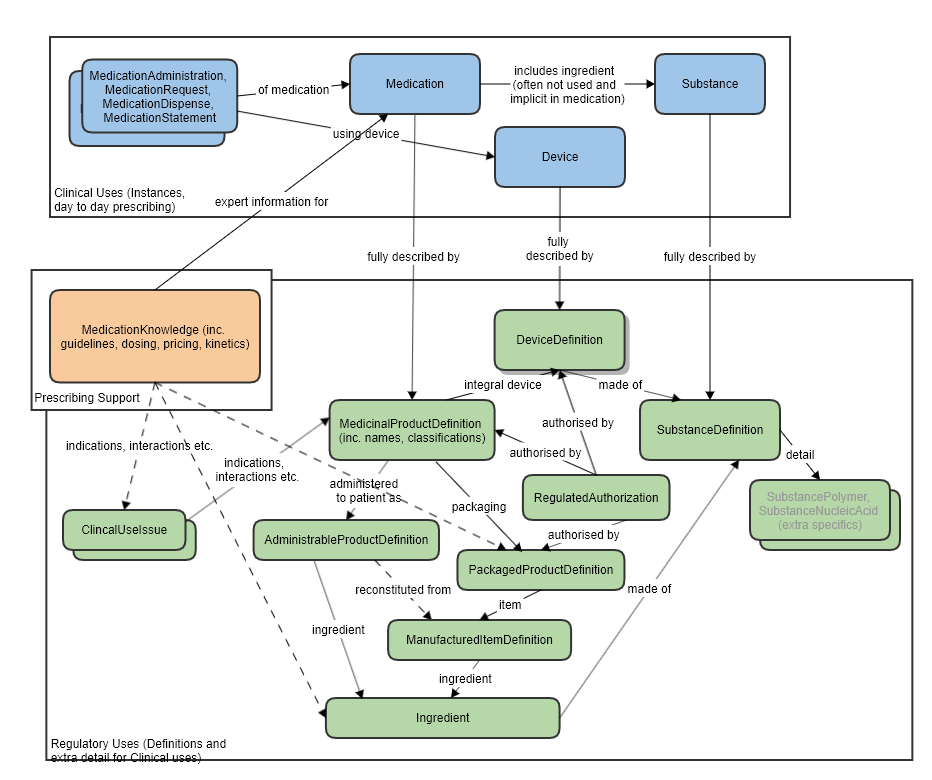
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): [[MedicationKnowledge_FHIR_Resource_Proposal]] | Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): [[MedicationKnowledge_FHIR_Resource_Proposal]] | ||
Latest revision as of 20:47, 4 September 2019
Contents
- 1 Ingredient
- 1.1 Owning work group name
- 1.2 Committee Approval Date:
- 1.3 Contributing or Reviewing Work Groups
- 1.4 FHIR Resource Development Project Insight ID
- 1.5 Scope of coverage
- 1.6 Resource appropriateness
- 1.7 Expected implementations
- 1.8 Content sources
- 1.9 Example Scenarios
- 1.10 Resource Relationships
- 1.11 Timelines
- 1.12 gForge Users
- 1.13 When Resource Proposal Is Complete
- 1.14 FMG Notes
Ingredient
Draft resource in build:
Owning work group name
Committee Approval Date:
6th May 2019 (earlier approval as "MedicinalProductAuthorization" 13th September 2017)
Contributing or Reviewing Work Groups
- Pharmacy
FHIR Resource Development Project Insight ID
1367
Scope of coverage
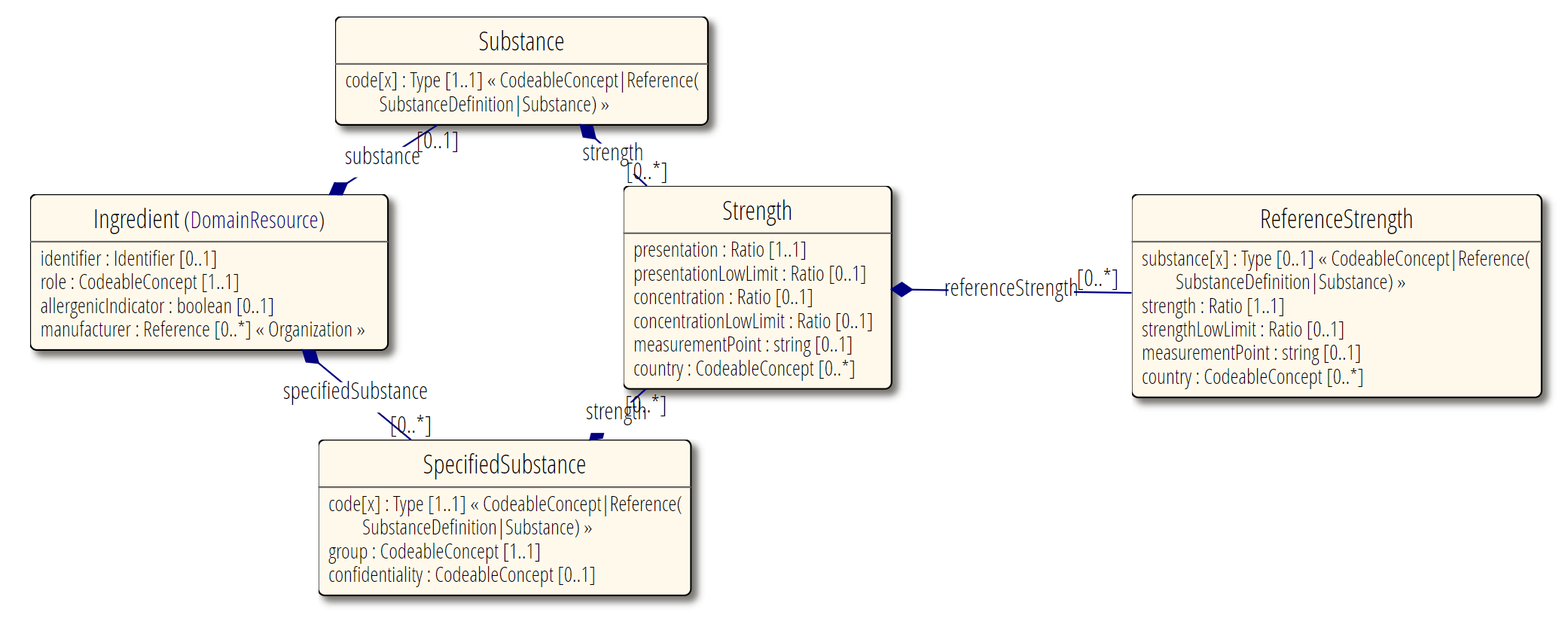
This resource covers the detail of pharmaceutical ingredients, to a more detailed level than exists in the Medication resource.
Both regulatory and medication knowledge use cases need structured ingredient and strength information to fully represent drug composition.
This includes:
Substance (including as one or more "specified" substance aspects including the grade or process)
Strength expressed in different possible ways (presentation, concentration) and details of how that strength it is measured.
Strength expressed as a function of a second substance (reference strength substance), for example as equivalent of a certain amount of the "salt".
Manufacturer.
Resource appropriateness
There is an outstanding requirement to support the standardised exchange of detailed "Product" data, for regulatory and other use cases.
This resource does not intend to clash with the existing Medication resource, but complements it with an extra level of detail.
Drug manufacturers currently submit this data electronically to regulators, when products are registered or altered, or marketing situations change.
This resource has been designed in close consultation with Pharmacy WG - Medication knowledge bases also cover this level of detail and this resource is currently referenced within the MedicationKnowledge resource
Expected implementations
EMA and European drug manufacturers, who have a requirement to submit to EMA (and already do so in a proprietary format). The EU wide system is currently being redeveloped to use FHIR, and this data is directly in scope.
FDA for Drug Submission (currently using SPL, which is not likely to change in the near term, but have expressed an interest in FHIR).
FDA for Pharmaceutical Quality (HL7 PSS approved, based on this resource, June 2019).
Content sources
The core basis for the resource is information currently used by FDA (as SPL) and the EMA EU XEVMPD data base (and XEVPRM XML messages).
Also, information gained from early stage implementation of this resources at EMA (2018, 2019), and from many many received to EMA about the draft API specification from the European medicines regulatory network (https://www.ema.europa.eu/en/about-us/how-we-work/european-medicines-regulatory-network).
Example Scenarios
Pharma companies submit details of new products to regulators, and include ingredient details.
Pharmacies and prescribers can view and download this information for reference and integration with their systems.
Decision support systems can use ingredient substance information for allergy checking.
Specific use cases include:
Submission of products from drug companies and NCAs (National Competent Authorities - the national regulators) to regional regulators.
This is already implemented in Europe (by EMA and EU-wide stakeholders) with an earlier non-HL7 format (XEVPRM/XEVMPD). That scenario is currently being re-implemented, using this resource, as part of the EU wide SPOR project.
Resource Relationships
See diagram below.
Some notable resource references:
To a manufacturing organization. Incoming relationships from the pharmaceutical products that use the ingredient.
Also refer to the logical model which was used to clarify the resource relationships, at the request of FMG, in the preparation of this proposal (linked to the approved MedicationKnowledge proposal page): MedicationKnowledge_FHIR_Resource_Proposal
High level relationships of the main prescribing resources and the regulatory strata below:
Timelines
Draft content is modelled in the FHIR build (http://build.fhir.org/regulatedauthorization.html), with outline supporting documentation. Completion planned Q4 2019.
gForge Users
riksmithies
When Resource Proposal Is Complete
When you have completed your proposal, please send an email to FMGcontact@HL7.org