Deprecating Lot Number Text
This is a process that was started at the CPM quarter at the San Antonio WGM January 2012 to explore how to manage the recording of "lot numbers" correctly, without using the lotNumberText attribute - which is noted in the CPM as being deprecated.
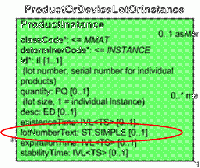
In CPM there is representation of an instance of a product, using R_ProductInstance (POCP_RM020100UV). The text for the instance says: A product instance is (a) an individual product instance, i.e., an individually identified thing with a serial number; (b) a product lot, i.e., a set of things of the same kind (e.g., 1000 for expressing a number of tablets) or an amorphous mass (e.g., 100 L of a liquid raw material), or (c) any subset, member, or portion of a product lot. The distinction between an individual thing and a lot is made in the quantity attribute, which is set to 1 (one) for individual items, or any number e.g., 1000 or amount 100 L for sets of things or amorphous masses respectively.
The entity that represents the product instance has the attribute lotNumberText, but this has the following design comments: <<DEPRECATED: The lot number for this instance. It identifies an amount of the product that has been produced or otherwise processed together. This attribute should NOT be used any more, as a product lot is represented as an instance of itself, and the individual (serialized) items, or sub-portions of the lot are represented as "members" of the lot instance.>>
The main business requirement is to be able to describe the lot number of an instance of a product - for example when a particular instance has caused an adverse reaction. This is the product instance as in example a) above, but with the proviso that not every type of product has an individual serial number. Many devices - and particularly implantable devices - will have an individual instance serial number which can be correctly described iin the id attribute using a root OID and extension - an perhaps also with a reliability identifier also. But for those products that do not have an individual instance serial number but which do have a lot or batch number (medicines, mass-produced devices such as syringes etc.) how should the id attribute and the II datatype be used to describe this information?
The Instance Identifier (II) datatype is made up of several parts - the most important from this perspective being the ROOT and the EXTENSION; the reliability indicator may have some business value. The root part contains a unique identifier that guarantees the global uniqueness of the instance identifier. There are cases where the root alone may be the entire instance identifier. The root is essentially the "namespace" of the identifier given in the extension and is itself identified by a UID - a Unique Identifier String. There are a couple of different types of UID in use currently - ISO Object Identifiers (OIDs) and DCE Universally Unique Identifiers (UUID). The extension part is a character string that serves as a unique identifier within the scope of the identifier root. So it is the root + the extension that give the global uniqueness.
Lot numbers and batch numbers are not currently globally unique, and currently few if any lot/batch numbers have any UID associated with them. However this is something that is very likely to change in the foreseeable future, and indeed in some domains is already changing, as various initiatives come on stream and with the availability of supporting technologies, even to the extent of mobile phones having bar-code reading apps that can gather unique lot/batch number as part of the GS1 product identification.
But in the meantime, a compromise must be found, which must also be able to deal with lot/batch number information reported in adverse events which may not be accurate or verifiable even within there non-unique limits (e.g. transcription errors or incomplete information).
The following are the scenarios to be managed: 1) Products that have a globally unique lot/batch number, with a root and an extension, which is, as part of the general clincial process, always recorded - such as implantable devices. These can be easily communicated in the id attribute using the II.