Difference between revisions of "Canonical Pedigree Project"
Scott.Bolte (talk | contribs) m (Present scenario 3 using new layout with pedigree) |
Scott.Bolte (talk | contribs) m (attribution) |
||
| Line 58: | Line 58: | ||
The need for detailed family health history can easily be demonstrated. For example, the chart for a 30 year old woman may have the statement "''family history of breast cancer -- mother and two aunts''". Unfortunately that simple statement is insufficient to distinguish between normal and increased risk. | The need for detailed family health history can easily be demonstrated. For example, the chart for a 30 year old woman may have the statement "''family history of breast cancer -- mother and two aunts''". Unfortunately that simple statement is insufficient to distinguish between normal and increased risk. | ||
| − | + | All three of the following scenarios fit the original statement. Pedigrees and risk scores courtesy of [http://www.hughesriskapps.com Hughes Risk Apps]. | |
<!-- ------------------ Scenario 1 ------------------> | <!-- ------------------ Scenario 1 ------------------> | ||
Revision as of 19:37, 7 December 2010
Contents
Canonical Pedigree Project Overview
The Canonical Pedigree Project (CPP) was proposed and approved at the HL7 Phoenix meeting of the Clinical Genomics work group in January, 2010. It is intended to improve adoption of the standard V3 pedigree message. It has three aspects:
- Reference Pedigrees: Provide reference pedigree messages with corresponding text descriptions of the family history. Intended to be a resource for family history collection software verification.
- Interoperability Testing: The internal storage of a pedigree is up to the host system. Furthermore, some interoperability standards want to represent a pedigree using alternate formats (e.g. CCD, vMR, clinical statements, etc.). The canonical pedigree project shall provide test guidance to verify that host systems and alternate formats are able to accurately maintain the relationships in the reference pedigrees. If full fidelity cannot be maintained, the guidance will help quantify the lost of fidelity.
- Clinical Power: Many systems provide support only for simplified family histories. For example, they will capture that there were two instances of aunts with breast cancer. That simplified perspective is in contrast with one that maintains maternal vs. paternal line, the number of available aunts and clinical details such as age of onset. The intent behind this facet of CPP is to quantify the clinical benefits of improving the granularity of family and clinical histories.
To learn more about the goals or status of CPP contact the project leader, currently Scott Bolte of GE Heatlhcare.
Interoperability Problems
Technical Equivalence
The adoption of the HL7 Pedigree standard message is inhibited by a lack of interoperability testing. That has lead to multiple systems that are generating incompatible messages. While wider use of the HL7 provided message schema would partially address that problem, there is still a problem verifying when messages are equivalent. Without requiring detailed knowledge of XML, the markup language used to capture a pedigree message, the following examples illustrate the problem.
- Well-Formed: An XML message is said to be well-formed if it conforms to the high-level XML syntax rules. For people unfamiliar with XML, here is a more familiar example of a street address for a letter that would be considered well-formed:
80 Old Faithful John Ranger
- Valid: An XML message is valid if it conforms to a schema definition that dictates the allowed content and ordering of elements. In the previous example, though a person may be able to guess how to send the letter to Mr. Ranger with its well-formed address, the address is actually invalid. The street element improperly comes before the person name element and the state & zip code elements are missing. An address that is both well-formed and valid according to generally accepted schema rules in the United States is:
John Ranger 80 Old Faithful Trail Yellowstone National Park, WY, 82190
- Equivalent: For interoperability, it is not sufficient that pedigree messages are valid. It is critical to be able to test if two pedigree messages are equivalent. Here are two valid addresses that are subtly different:
John Ranger Old Faithful Visitor Center 80 Old Faithful Trail Yellowstone National Park, WY, 82190
John Ranger 80 Old Faithful Trail Yellowstone National Park, WY, 82190
It is central to the canonical pedigree project to be able verify generated pedigree messages are equivalent to the reference messages.
Degrees of Equivalence
There are three levels of equivalence testing that needs to be done.
- First, there is the comparison of two messages where spaces, carriage returns, encoding (e.g. '<' vs. <) is standardized. This should be fairly straightforward.
- Second, there is comparison where attributes or elements are sorted differently. For example, one system might list the people from oldest to youngest while another does the reverse. Both lists are equivalent, but it is harder to confirm the XML structures are equal.
- Finally, there are semantic equivalents to test. Niece is equivalent to sister's daughter, but coming up with a system that can correctly discern that will be a challenge.
Reference Pedigrees
The following sample is an elaborated pedigree that supplements the standard specification: Patient has two sisters, a husband a daughter, and a mother and a father (each has two parents): Media:PedigreeSampleElaborated.doc
Clinical Power
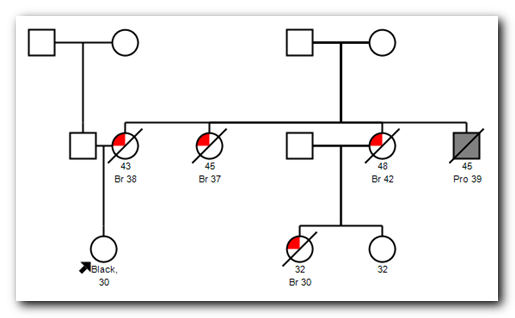
The need for detailed family health history can easily be demonstrated. For example, the chart for a 30 year old woman may have the statement "family history of breast cancer -- mother and two aunts". Unfortunately that simple statement is insufficient to distinguish between normal and increased risk.
All three of the following scenarios fit the original statement. Pedigrees and risk scores courtesy of Hughes Risk Apps.
If you compare scenarios 1 and 2, it is quite clear that the age of onset of the disease shifted from the 60's to around 40. Similarly, scenario 1 had the incidences of cancer split across family lines, and amongst a large group of siblings. In contrast, scenario 2 was all on one side, and 100% disease penetration included both the three sisters and their brother. A knowledgeable clinician will recognize the first scenario is likely just sporadic cancers that are not terribly surprising given the patients' ages while the second shouts out there is a genetic component.
Now the first two scenarios were crafted to highlight the hazard of drawing conclusions from a simplified family history. However, it is the third situation that is more likely, and more difficult to assess without decision support. By the time 3rd degree relatives are included it becomes quite difficult to assess risk by hand. However, the high clinical value to patients makes it clear that collecting, analyzing, and acting upon a detailed structured family history is worth improving.