Product RR aECG
Product Brief - V3 Regulated Studies: Annotated ECG (aECG)
Contents
- 1 Product Brief - V3 Regulated Studies: Annotated ECG (aECG)
- 1.1 Product Name
back to Main_Page
back to Product_List
Product Name
Annotated ECG (aECG)
Topics
- Annotated ECG
Standard Category
- Health Information Exchange Standards
Integration Paradigm
- Messages
Type
Normative, ANSI Standard
Releases
ANSI/HL7 V3 ECG, R1-2004 (ANSI Reaffirmation 2009)
Summary
The Annotated ECG (aECG) HL7 standard was created in response to the FDA’s digital ECG initiative introduced November, 2001. The FDA continues to be concerned about evaluating noncardiac drugs for negative cardiac effects, like prolonged QT. Before this initiative, sponsors were already submitting ECG findings tabulations (e.g. QT interval measurements) with their applications. However, the FDA could not systematically evaluate the ECG waveforms and measurement locations those findings came from. Most (if not all) ECGs in current trials were collected with paper and not electronically retained. The next logical step for the FDA was to ask that the digital waveforms and measurement locations (annotations) be made available with the application.
A necessary step for submitting the ECG waveforms and annotations to the FDA was to have a standard format for the data. An evaluation of current ECG waveform standards found no existing standards that met all the FDA’s needs. Therefore the FDA, sponsors, core laboratories, and device manufactures worked together within HL7 to create a standard to meet the needs.
The aECG standard was created by HL7’s Regulated Clinical Research Information Management (RCRIM) in response to the FDA’s need.
Description
The FDA continues to be concerned about evaluating noncardiac drugs for negative cardiac effects, such as prolonged QT. Before this initiative, sponsors were submitting ECG findings tabulations (e.g. QT interval measurements) with their applications. However, the FDA could not systematically evaluate the ECG waveforms and the measurement locations the findings came from. Most (if not all) ECGs were collected with paper and not electronically retained. HL7’s Regulated Clinical Research Information Management (RCRIM), in response to the FDA’s need, created the Annotated ECG (aECG) standard.
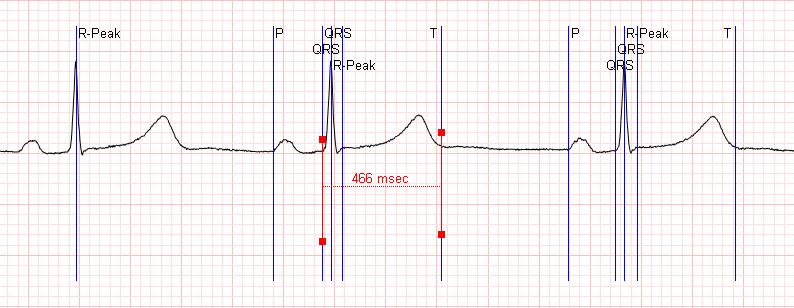
THE PURPOSE OF THE aECG
The purpose of the aECG message is to provide a means to systematically evaluate the ECG waveforms and measurement locations.
The most recent version, Release 1, received ANSI approval in May 2004 and was reaffirmed in May of 2009. It is available for purchase at the HL7 bookstore at www.HL7.org as a part of the current v3 Normative Edition
Business Case (Intended Use, Customers)
Following the development of the standard, an ECG Warehouse (www.ecgwarehouse.com) was created for the collection and storage of protocol driven annotated ECGs. The E-Scribe ECG Warehouse, a public-private collaboration with the FDA, provides tools for annotated ECG review, scoring and warehousing. Sponsors and central laboratories upload annotated ECG datasets supporting new drug applications to FDA into the E-Scribe ECG Warehouse to facilitate regulatory review using the toolset provided within the warehouse. The data upload process features a validation step to verify all annotated ECGs adhere to the HL7 annotated ECG standard.
Today there are more than three million annotated ECGs stored within the E-Scribe ECG Warehouse. They represent the largest collection of standardized ECGs in the world. The FDA hopes to mine this vast collection of ECGs to accelerate the investigation of improved biomarkers and to better characterize the cardiac safety of new drugs.
HL7 Annotated ECG is now the most widely adopted ECG standard in the world. All device manufacturers and software companies having anything to do with ECGs, and the pharmaceutical industry, support this format.
Benefits
The standard provides a common means of electronically storing both the ECG wave form and associated annotations. This allows for comparative analysis in the assessment of pharmaceutical products for negative cardiac effects.
Implementations/ Case Studies (Actual Users)
US FDA, Pharmaceutical companies, Clinical Research Organizations
Resources
www.ecgwarehouse.com
http://www.fda.gov/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/ucm085324.htm - See section on Providing Digital Electrocardiogram (ECG) Data
Work Groups
Education
- See more at http://www.hl7.org/implement/training.cfm
Links to current projects in development
- Project Insight ID # 462, Annotated ECG, Release 1 ANSI reaffirmation